Keywords: peptide; peptide hydrolysis; peptide bonds; peptide modifications; peptide synthesis; peptide bond; peptide hormones; peptide analogues; salting out; amidation; acetylation
Peptides
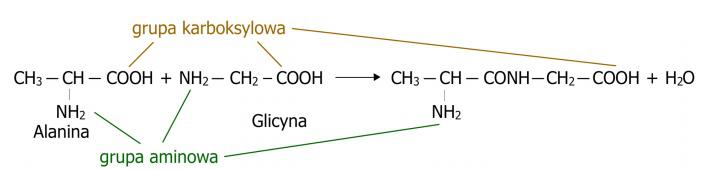
Peptides are chemical compounds constructed similarly to proteins, from amino acids. They are formed by combining two or more amino acids using a peptide bond as a result of a condensation process, where, in addition to the peptide, a water molecule is also formed. (Fig. 1) They are the subject of widespread interest, performing important biological functions. Many hormones and neurotransmitters are peptides. In the case of endogenous peptides, they have an antimicrobial effect, acting as the body's defence system. Naturally occurring peptides and their synthetic analogues are considered attractive compounds of therapeutic importance due to their high degree of activity, low toxicity and lack of interaction with drugs. In medical practice, only a few peptides are used due to their biological instability and rapid decay, but peptide synthesis allows stable forms to be obtained. The same is true, for example, in the case of peptide synthesis from natural sources. Peptides occur in an unbranched form and have only two specific ends. One of them is called the amino end, where there is an amino acid with a free α-amino group. The other is called the carboxyl end or C end, where there is an amino acid with a free α-carboxyl group.

Figure 1. Illustration of the condensation process, i.e. the process of peptide formation.
Nomenclature of peptides
The naming of peptides begins with the name of the N-terminal amino acid residue, followed by the names of the subsequent amino acid residues, and ends with the name of the C-terminal amino acid. The sequence of amino acids is written using three-letter or single-letter symbols.
Peptide bond
As a result of the reaction of the α-carboxyl group, carbon binds to the nitrogen of the α-amino group via a single bond, a peptide bond. It is assumed that this bond is formed in the form of two structures that remain in a specific mutual equilibrium. The C-N bond changes to C=N and vice versa. Rotation relative to the C=N axis is not possible, which makes the peptide bond so rigid that it has the characteristics of a double bond. In the case of a peptide bond involving the imine group of proline or hydroxyproline with the carboxyl group of another amino acid, a different, separate structure is formed. In this case, nitrogen is incorporated into the pyrrolidine ring structure, there is no hydrogen substituent, and therefore no rotation is possible relative to the bonds that form in the presence of nitrogen. Amino acids involved in the formation of a peptide bond lose fragments of their molecules. These are -OH molecules from the carboxyl group and -H molecules from the amino group. Therefore, amino acids found in peptides and proteins are called amino acid residues. The resulting peptide bonds are stable and can only be broken down by strong bases and acids at high temperatures. The formation of a peptide bond is shown in the diagram. (Fig. 2)

Figure 2. Diagram of peptide bond formation
Peptide bond cleavage
The breaking of peptide bonds occurs as a result of peptide hydrolysis, which involves the breaking of the peptide bonds that have been formed and the recreation of individual amino acids. Water participates in this reaction, its molecules breaking down into hydroxyl groups (-OH) and hydrogen atoms (H), which then combine with the released bonds of the substance. The diagram shows the peptide hydrolysis reaction. (Fig. 3)

Figure 3. The hydrolysis process causing peptide bond cleavage
Classification of peptides
Peptides are classified according to the number of amino acids they contain. In the general classification of peptides, we distinguish between:
- Dipepetides – products formed from the reaction of two amino acids, retaining the free amino group of one amino acid and the free carboxyl group of the other amino acid;
- Oligopeptides – peptides composed of several to a dozen or so amino acids;
- Polypeptides – longer peptides containing several dozen amino acid residues;
- Proteins – accepted when a molecule consists of more than one hundred amino acid residues.
Peptide formulas
Peptide formulas are created based on a table containing protein amino acid formulas and their abbreviations (Table 1).

Table 1. Amino acid formulas used for naming peptides
Spectrum of peptide activity
Peptides exhibit a wide spectrum of biological activity and are used in the treatment of bacterial infections, viral diseases, cardiovascular diseases, skeletal diseases, nervous system diseases, diabetes and osteoporosis.
Advantages of peptides
- High activity and selectivity
- Wide range of molecular targets
- Potentially lower toxicity compared to low molecular weight compounds
- Low accumulation in tissues
- High chemical and biological diversity
- Possible to discover at the gene level
- Easy synthesis of analogues
Peptide synthesis
Depending on the peptide we want to obtain, we need an appropriate method for its synthesis. In this brief explanation, we will try to present peptide synthesis in relation to its size. To obtain a dipeptide, use a reagent that will activate the carboxyl group of the acylating amino acid or convert the acylating amino acid into an anhydride. A more labour-intensive and difficult process is the synthesis of larger peptides, which we obtain from a dipeptide, where the amino group of the N-terminal amino acid is removed and acylated with another N-protected amino acid. This process is particularly time-consuming because the above steps are repeated until a peptide with the planned sequence is obtained. In the case of obtaining large peptides, the Merifield method is the most effective and easiest method. This method is carried out in the solid phase. The C-terminal amino acid is attached to the polymer and then another amino acid is attached until the desired chain length is achieved.
Biologically active peptides
Peptide hormones and protein hormones are commonly found in our environment. Previously known mostly as unstable forms, thanks to synthesis, it is now possible to select peptide therapies that are long-lasting and effective, depending on the body's needs. That is why it is worth skilfully and safely experimenting with hormone stimulation. Considering some biologically active peptides, we can give the example of glutathione, which is a tripeptide with a specific structure composed of glutamate, cysteine and glycine. Glutamate occurs as an N-terminal amino acid. However, the combination of glutamate and cysteine is unusual for peptides and proteins, as there is no α-carboxyl group of glutamate here, only a γ-carboxyl group. Glutathione therefore occurs in reduced and oxidised forms, being γ-glutamylcysteinylglycine. In its reduced form, it has a free sulfhydryl group, and in its oxidised form, a pair of hydrogen atoms detaches from the –SH groups. The sulphur atoms remain devoid of hydrogen, resulting in the formation of a disulphide bridge. The ability of glutathione to be modified into an oxidised or reduced state is important in oxidation-reduction processes.
Another example is oxytocin and vasopressin, which are nanopeptides produced by neurons in the hypothalamus and released by the posterior lobe of the pituitary gland, differing only in two amino acids. Cysteine occurs in two positions, leading to the formation of a disulphide bridge. Oxytocin acts as a hormone that stimulates uterine contractions. Vasopressin, on the other hand, stimulates water absorption in the renal tubules. Vasopressin also plays an important role in regulating the secretion of adrenocorticotropic hormone (ACTH) in stressful situations.
Peptide hormones
Adrenocorticotropic hormone (ACTH)
Adrenocorticotropic hormone, a 39-amino acid peptide, is formed as a result of the degradation of a much larger precursor molecule, proopiomelanocortin (POMC). Proopiomelanocortin is also a source of other active peptides. Two peptides are contained in the ACTH structure. These include α-melanocyte-stimulating hormone (α-MSH), which is structurally identical to the first 13 amino acids of ACTH, and a peptide from the intermediate pituitary gland similar to corticotropin-fragment 18-39 ACTH. The primary function of ACTH is considered to be the stimulation of the adrenal cortex so that it is able to secrete steroid hormones. Adrenocorticotropic hormone is responsible for regulating activity at the level of the fascicular and reticular layers. The first 18 amino acids are responsible for the biological activity of ACTH. ACTH is regulated by corticotropin-releasing hormone (CRH), a hormone found in the hypothalamus, which releases corticotropin via cortisol through negative feedback. This means that cortisol deficiency stimulates CRH and ACTH, while its excess inhibits secretion. Thus, the release of cortisol regulates many important vital functions, including the mobilisation of the body to stressful conditions, increased blood pressure and anti-inflammatory capabilities. ACTH is secreted in pulses in a circadian rhythm, meaning that its highest concentration is observed in the morning when it is most needed, and then decreases as the day progresses. An increase in ACTH secretion is observed in conditions such as adrenal insufficiency, Cushing's disease and Nelson's syndrome.
Insulin and C-peptide
Insulin and C-peptide are secreted in the pancreas by the human body all the time. During insulin production, in the process of its biosynthesis, C-peptide is produced. Pancreatic cells first produce peproinsulin, which undergoes further modification through the removal of amino acids, resulting in the formation of proinsulin composed of two chains, A and B, which are linked by C-peptide. Next, C-peptide is removed from proinsulin, resulting in the final form. When glucose appears in the body, the pancreas receives a signal to release granules with stored insulin and C-peptide molecules. C-peptide is retained in the liver much longer than insulin because it is not degraded there. Its breakdown occurs mainly in the kidneys. In the case of both insulin and C-peptide, elevated or too low concentrations lead to the development of type I or II diabetes as well as Cushing's disease. In the case of C-peptide, fluctuations in concentration may also indicate chronic renal failure or the presence of metastases or local tumour recurrence, which is why it is so important to maintain normal concentration levels.
Motilin
Motilin is a hormone associated with the smooth muscles of the stomach and intestines, controlled by the vagus nerve fibres. It is synthesised in endocrine cells. As a peptide hormone consisting of 22 amino acids arranged in a specific sequence, it is produced by cells in the small intestine. Produced by endocrine cells of the digestive system M (Mo), it participates in the regulation of gastrointestinal motility. Motilin is an important hormone involved in the formation of phase III of the migrating motor complex (MMC), in which the stomach and small intestine are responsible for emptying the stomach of unnecessary food residues and exfoliated epithelial cells by stimulating peristaltic movements. The hormone also influences the emptying of the gallbladder during the interdigestive period when motilin concentration is at its highest.
Glucagon
Glucagon is one of the hormones involved in regulating glucose concentration. This peptide is secreted by the endocrine cells of the pancreas. It is a polypeptide composed of 29 amino acids, formed from a precursor consisting of 180 amino acids. Changes in glucose concentration allow glucagon to be secreted. The production of glucagon occurs in the pancreatic islets, where proglucagon is converted into glucagon and glucagon-related polypeptide (GRPP). The main task of glucagon is to maintain normal serum glucose levels when they drop between meals or during physical exertion. In such situations, its reserves are released from the liver to provide the body with adequate protection. In addition, it can participate in regulation during food intake, which can make you feel full sooner. Glucagon can potentially inhibit the release of ghrelin and also inhibit intestinal peristalsis.
Peptide analogues
Peptide analogues are chemical compounds in which one atom is replaced by another in relation to the parent compound. The overall structure of the peptide remains unchanged. Peptide analogues include helical analogues and β-turn and β-sheet analogues. In the former, helices are one of the key structural elements of bioactive peptides. Stabilising short oligomer fragments in a helical conformation increases activity. In β-bend and β-sheet analogues, D-amino acid or β,γ,δ-amino acid residues are inserted. Peptide analogues allow us to obtain new peptide compounds that will be more stable, find application in a wider range of symptoms, and enable innovative solutions to problems associated with the operation of existing pre-analogue forms.
Peptide desalting
The desalting process involves changing protein charges. Protein charges are neutralised by salt anions and cations. Protein molecules do not attract each other and do not form aggregates, and the protein itself is precipitated as a result of losing its water coat. The desalting process is reversible. In the reversal process, the salt is removed by dialysis or its concentration is reduced by adding water. Based on our previous articles, it can be safely concluded that salting, which led to the creation of a stable form of the BPC-157 peptide, is an innovative method of ensuring peptide stability and, consequently, extending the biological activity of peptides.
Acetylation of peptides
Acetylation involves the attachment of acetyl radicals to substrates, which are compounds with NH₂, OH or SH groups, with the participation of the enzyme N-acetyltransferase. The source of the acetyl radical is acetyl-CoA. The main function of N-acetyltransferases is to facilitate the attachment of the acetyl group to the amino group of aromatic amines and hydrazines (N-acetylation reaction), i.e. the detoxification of potentially toxic exogenous compounds.
Amidation of peptides
When peptide bonds are broken and the polypeptide chain is fragmented, carbonyl groups are formed. The oxidation of a protein molecule by a hydroxyl radical begins with the removal of a hydrogen atom from the α carbon of an amino acid. The resulting alkyl radical reacts with oxygen to form an alkyl peroxide radical, which is converted into an alkyl hydroperoxide. The alkoxyl radical formed from it can be converted into a hydroxylated amino acid residue at the α carbon or can lead to fragmentation of the polypeptide chain. The presence of an alkoxyl radical promotes fragmentation of the polypeptide chain. Peptide bond cleavage can occur via α-amidation or diamidation. The N-terminal peptide formed during α-amidic fragmentation has an amide group at the C-terminus, while the other peptide contains an N-α-ketoacyl derivative at the N-terminus. Diamide fragmentation is characterised by the formation of an N-terminal peptide containing a diamide structure and a peptide derived from the C-terminal of the protein molecule containing an isocyanate structure at the N-terminal.
Bibliography
1.Murray R. K., Granner D. K., Mayes P. A., Rodwell V, Biochemia Harpera. 1995; Wydawnictwo Lekarskie PZWL 2.Jakubke H. D., Jeschkeit H, Aminokwasy peptydy białka. 1982; Państwowe Wydawnictwo Naukowe 4.Kołodziejczak A, Aminokwasy i peptydy. 2006





The effect of BPC-157 therapy on the immune system
Peptides with antimicrobial properties and their analogues created through modification.