TECHNOLOGY AND RESEARCH
RESEARCH, PATENTS & TECHNOLOGY
The New Generation of Peptides: NL-PEPTIDES™ + NL-PEPTIDES-DELIVERY™ Synthesized in Europe. Tested in accredited laboratories in Poland, Germany, and Switzerland. Result: greater stability, higher bioavailability, and predictable biological performance.
Purity, stability, and precision — these are the foundations of everything we create.
Every SYNTHAGEN product is developed using patented NL-PEPTIDES™ and NL-DELIVERY™ technologies, ensuring maximum bioavailability and efficacy.
We work through a transparent and rigorous research process, giving you confidence that you receive a product of verified laboratory quality.
This is not the industry standard — it’s the SYNTHAGEN standard.

Scientific Approach
SCIENCE STANDS BEHIND EVERY PRODUCT
Our NL-PEPTIDES™ and NL-DELIVERY™ technologies are patent-protected and are the result of years of research on peptide stability and absorption. Thanks to them, our products achieve higher biological effectiveness — unattainable in conventional formulations.
Each batch of SYNTHAGEN products undergoes independent testing in accredited laboratories in Poland, Germany, and Switzerland. We test purity, concentration, and composition consistency to ensure that every peptide meets the highest standards of safety and quality.
Our peptides are synthesized in Europe under strictly controlled laboratory conditions using certified raw materials and methods.
This is where the precisely engineered structure of each peptide is created — ensuring its stability, durability, and full biological activity.



NL-PEPTIDES
WHAT ARE NL-PEPTIDES™?
NL-PEPTIDES™ are next-generation analogues of classical peptides, engineered to retain their biological activity even under harsh physiological conditions — including gastric acids, digestive enzymes, and elevated temperature.
Through precise chemical modifications (N-terminal acetylation and C-terminal amidation), we have achieved peptide molecules with stability comparable to natural human proteins.
Their amino acid sequence remains intact, meaning that even after passing through the digestive tract, NL-PEPTIDES™ maintain their original structure and full biological activity.
✅ The result: peptides that remain effective not only via injection, but also in oral form — a milestone that was previously unattainable with traditional peptide structures.
Products
WHAT IS NL-PEPTIDES-DELIVERY™?
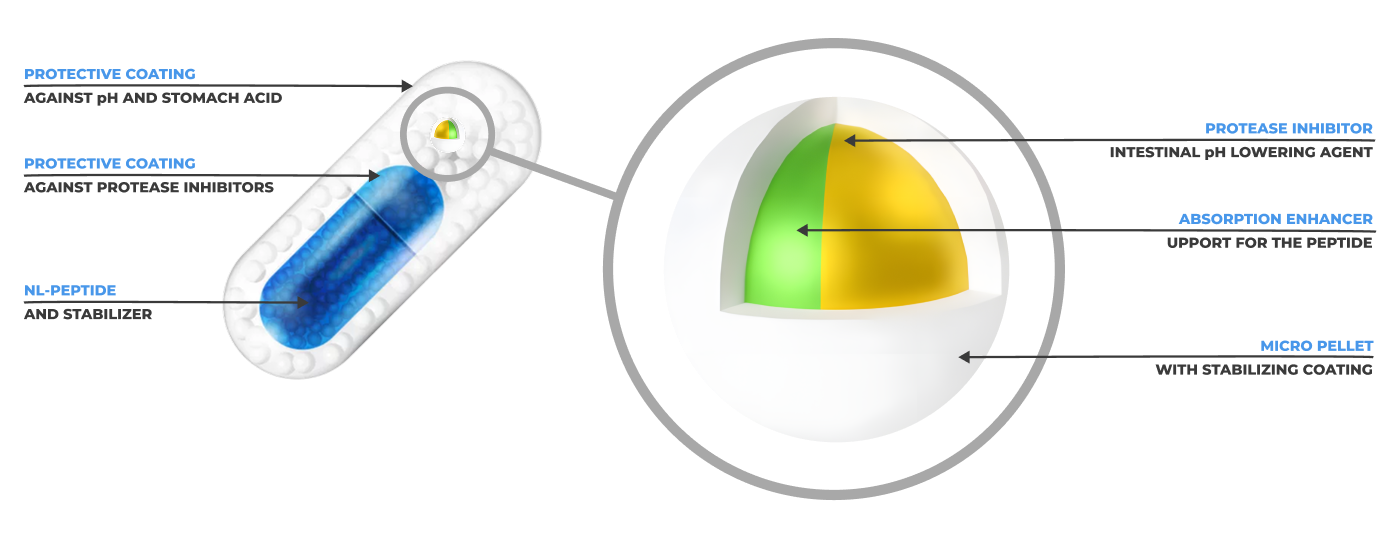
A Patented Dual-Capsule Technology for Effective Oral Delivery. NL-PEPTIDES-DELIVERY™ is our proprietary oral transport system, developed to solve two of the greatest challenges in peptide science: acidic degradation in the stomach and poor intestinal absorption.
The outer capsule is coated with an acid-resistant shell, allowing it to pass safely through the stomach and reach the small intestine intact.
Inside lies the micro-capsule core, containing: the active peptide, a protease inhibitor (to block enzymes that degrade proteins), and an absorption enhancer, which facilitates passage across the intestinal barrier.
This dual-layer technology ensures the peptide is fully protected until absorption, enabling complete biological activity once it enters systemic circulation.
📊 In vitro and in vivo studies confirm that NL-PEPTIDES-DELIVERY™ increases plasma peptide concentrations several-fold compared to standard capsule formulations.

EFFECT
A NEW STANDARD IN PEPTIDE TECHNOLOGY
By combining two patented innovations — NL-PEPTIDES™ and NL-PEPTIDES-DELIVERY™ — we created a system that solves three fundamental challenges in modern peptide pharmacology:
- Stability — resistance to external factors and digestive enzymes.
- Protection — no degradation in the stomach thanks to acid-resistant encapsulation.
- Bioavailability — complete absorption and verified presence in the bloodstream.
This is more than a technological breakthrough.
It is a redefinition of how peptides can be delivered safely and effectively in oral form, without losing their natural biological properties.
EP22156902
European patent covering the synthesis and stabilization of peptide analogues under the NL-PEPTIDES™ platform and their oral delivery system NL-PEPTIDES-DELIVERY™ — setting a new benchmark in peptide biotechnology.
DownloadAnalytical Report
Comprehensive purity, stability, and biological activity analyses of NL peptides, performed in accredited laboratories in Poland and Germany.
DownloadProduct Composition Study
Detailed chemical composition confirming molecular integrity and pharmaceutical-grade peptide synthesis.
DownloadNL-BPC-157 Functional Characterization
Cellular-level studies confirming regenerative efficiency, stability, and bioavailability.
DownloadNL-Epithalon Functional Characterization
Evaluation of telomerase activation, cellular regeneration, and biological aging delay.
DownloadNL-GHK-Cu Functional Characterization
Assessment of peptide-copper complex activity in collagen synthesis, skin regeneration, and antioxidant protection.
DownloadNL-Cortagen Functional Characterization
Studies confirming neuroprotective effects, DNA stabilization, and gene expression regulation.
DownloadNL-Selank Functional Characterization
Evidence of anxiolytic, mood-stabilizing, and cognitive-enhancing effects.
DownloadNL-Semax Functional Characterization
Analysis of nootropic and neuroregenerative properties — concentration, memory, neurogenesis, and oxidative protection.
DownloadChromatography & Mass Spectrometry (MS)
Analytical confirmation of purity, molecular weight, and structural integrity of base peptides.
DownloadChromatography & MS of NL-Peptides
Detailed molecular validation of NL-PEPTIDES™ structures, confirming pharmaceutical stability and conformity with reference standards.
DownloadSynthagen
RESEARCH AND PATENTS OF SYNTHAGEN LABS
All Synthagen Labs formulations and technologies are based on years of R&D, validated by patents, laboratory reports, and analytical testing.
Every product undergoes a comprehensive quality verification process — from synthetic purity and structural stability to bioavailability and biological safety assessments.
NL-PEPTIDES
Patented peptides
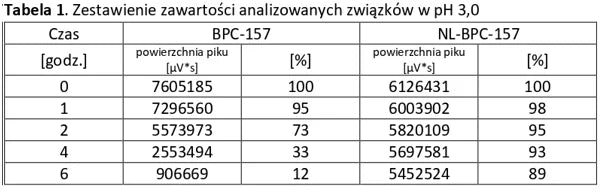
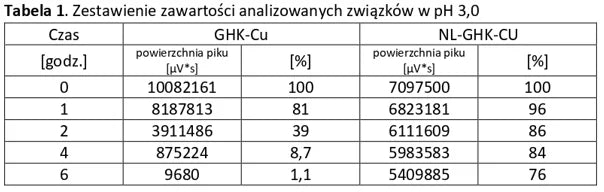
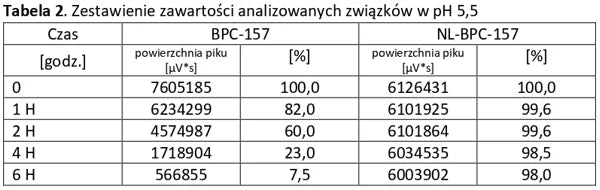
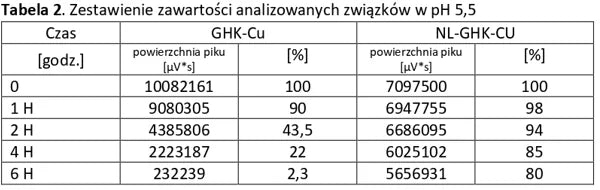
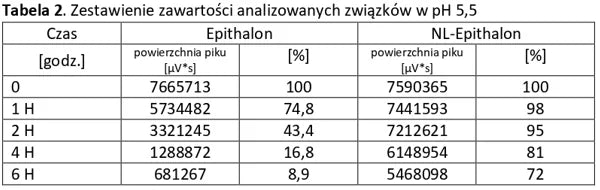
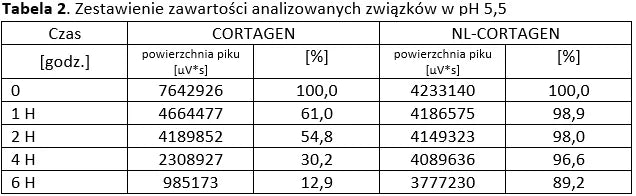
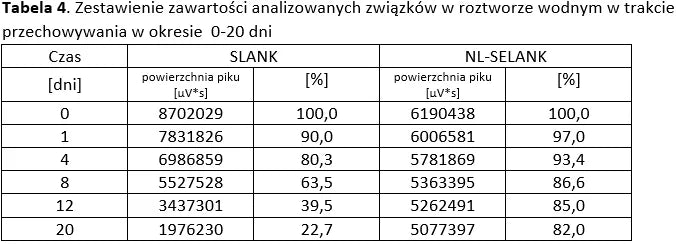
The tables below present a comparison between NL-peptides and regular peptides. The comparative studies were conducted in environments simulating the digestive system, as well as during peptide storage and in their dissolved form (vials).
NL-PEPTIDES – Your new, improved, patented peptides.
The tables below present a comparison between NL-peptides and regular peptides. The comparative studies were conducted in environments simulating the digestive system, as well as during peptide storage and in their dissolved form (vials).
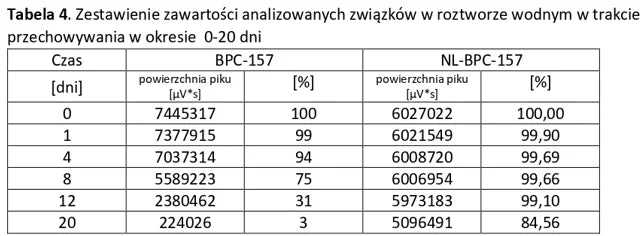
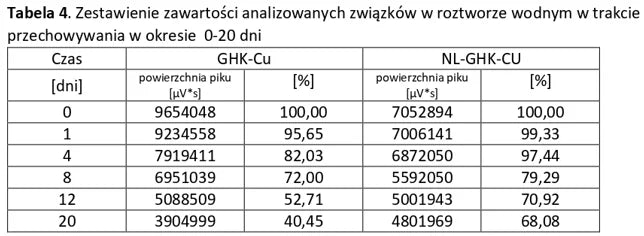
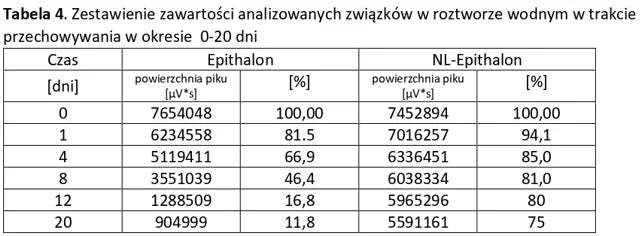
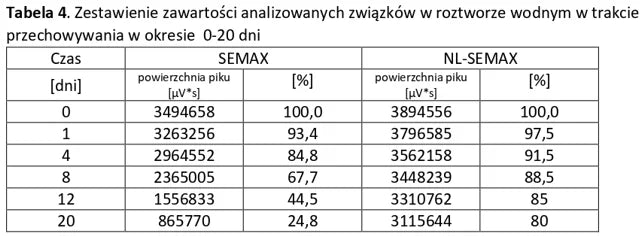
Conclusion: NL-PEPTIDES are significantly more stable than regular peptides, making them suitable for use in capsules and oral pharmaceutical formulations.
Analysis of basic peptides and NL-peptides in an environment simulating stomach conditions (pH 3).
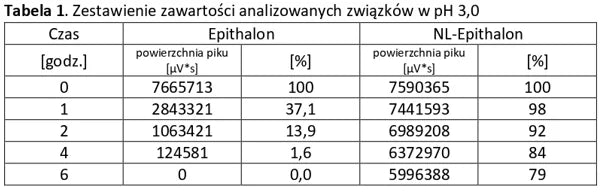
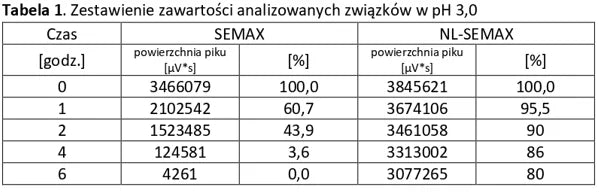
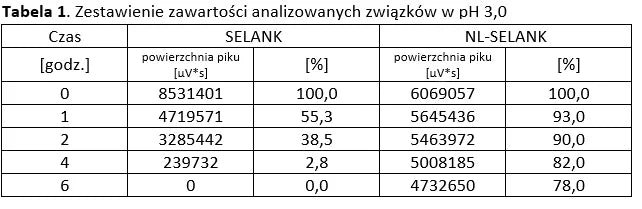
Analysis of basic peptides and NL-peptides in an environment simulating stomach conditions (pH 5.5).
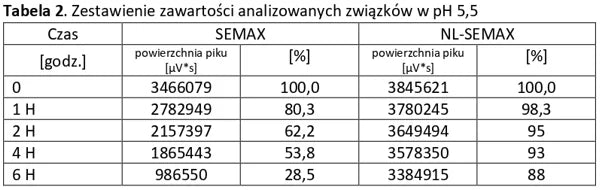
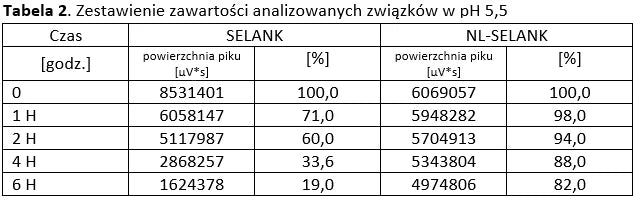
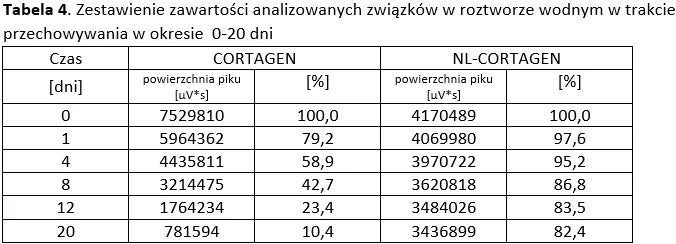
Stability of peptide lyophilizates during storage.
Samples of the lyophilizate sealed in airtight vials were stored for 365 days at 35°C and standard humidity. Analyses were performed by opening successive vials at one-month intervals.
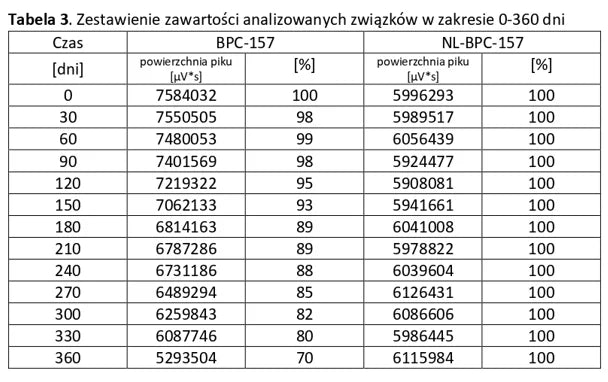
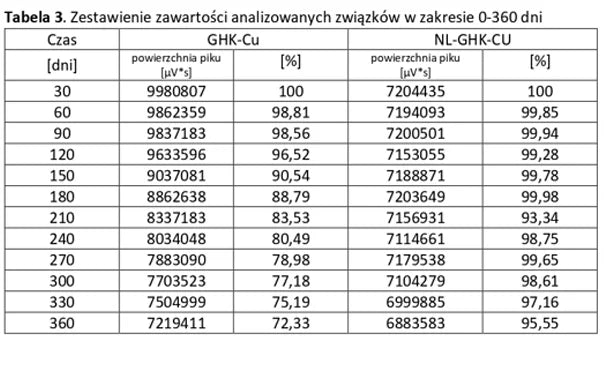
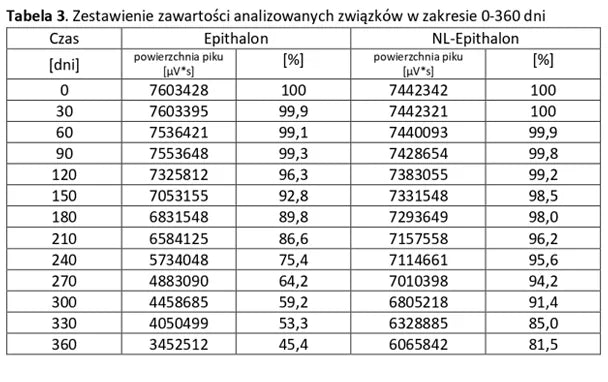
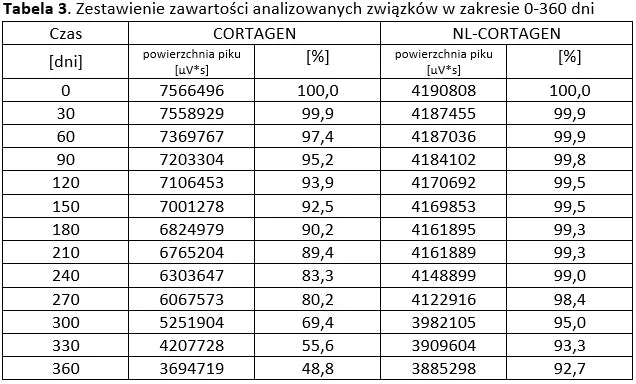
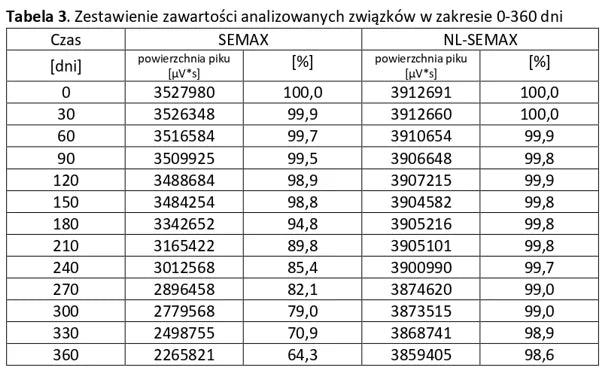
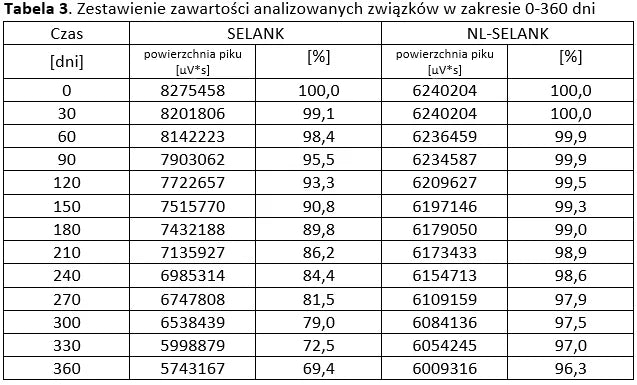
Stability of aqueous solutions during storage.
Structural stability was analyzed using peptide solutions prepared as follows: Peptide solutions with a concentration of approximately 1 mg/ml were prepared in demineralized water (pH around 5.6). The prepared solutions were stored under controlled conditions at 40°C and 55% humidity, with measurements taken at regular time intervals.

NL-PEPTIDES-DELIVERY™ – Your new, patented dual-capsule technology.
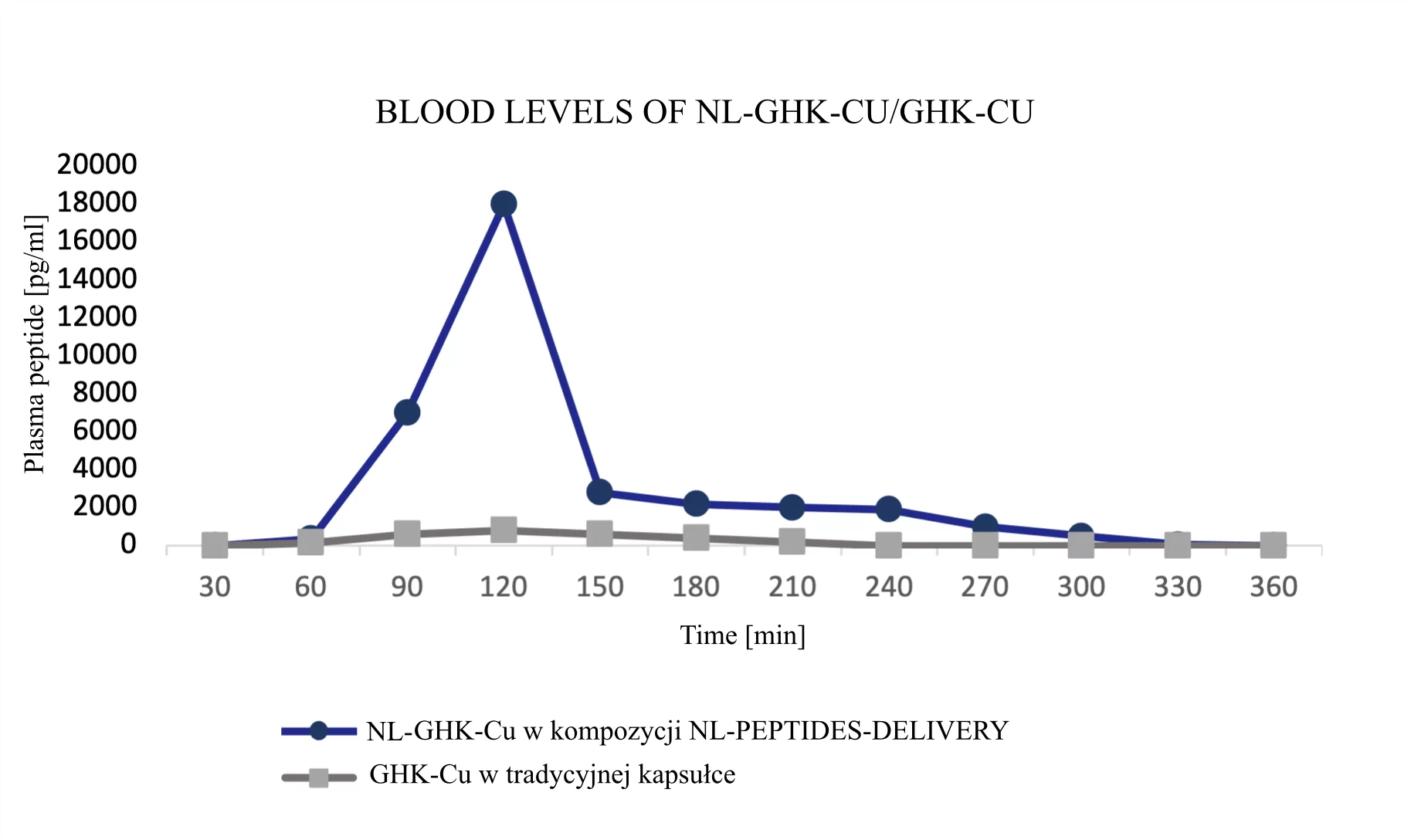
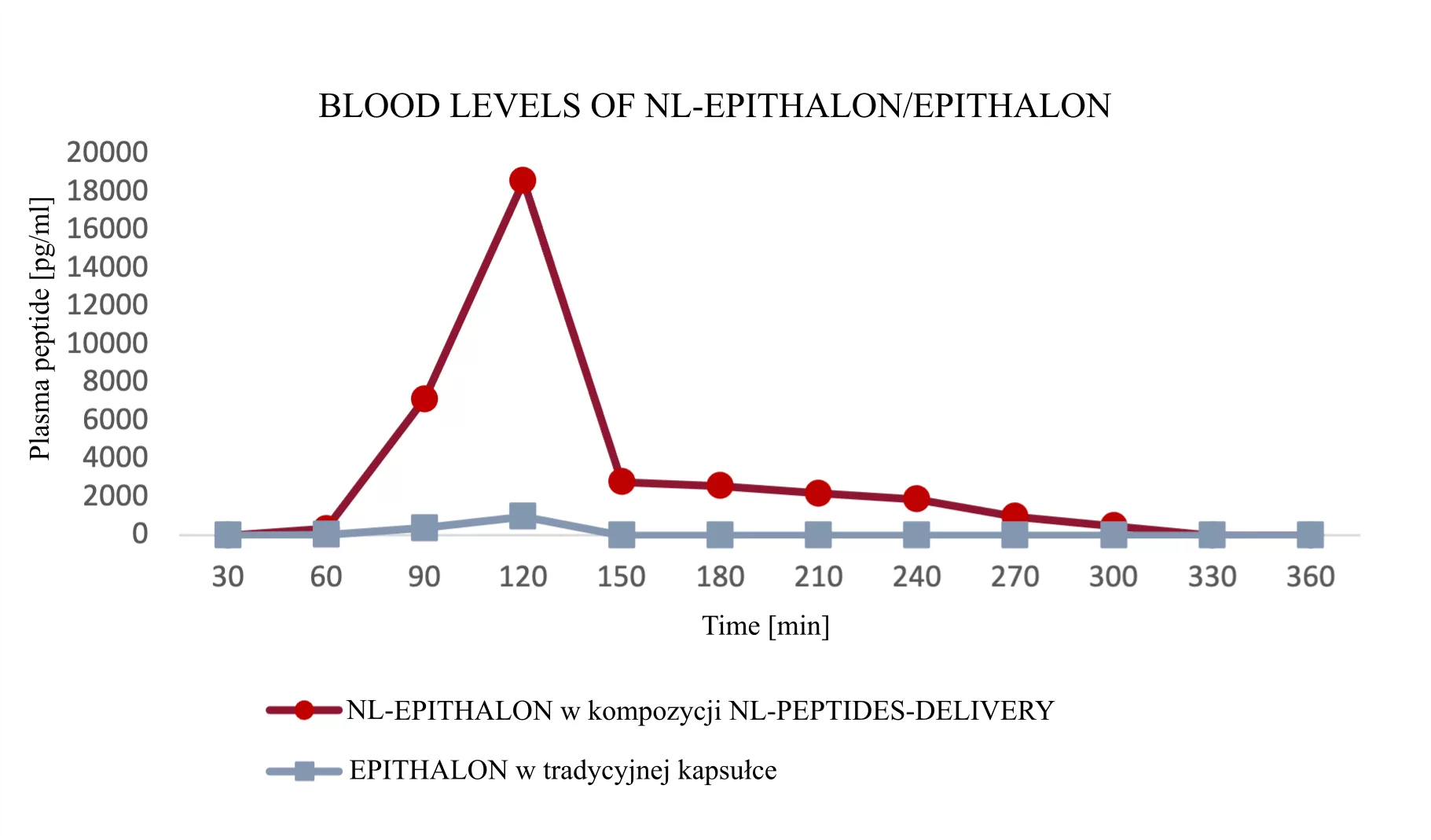
Results of NL-peptide concentration studies in blood
The first three example graphs show peptide peaks from appropriately prepared blood samples analyzed 120 minutes after administration. These correspond respectively to NL-GHK-CU, NL-EPITHALON, and NL-BPC-157 administered using our patented NL-PEPTIDES-DELIVERY™ technology, as well as regular peptides GHK-CU, EPITHALON, and ARG-BPC administered in standard capsules. The next three graphs illustrate the concentration profiles of peptides in blood measured over a time range of 30–360 minutes, expressed in pg/ml.
Conclusion: Thanks to our dual-encapsulation technology, oral administration of peptides has become possible. The graphs below demonstrate clear evidence of our technology’s effectiveness.
Source: EP22156902 and P22156902
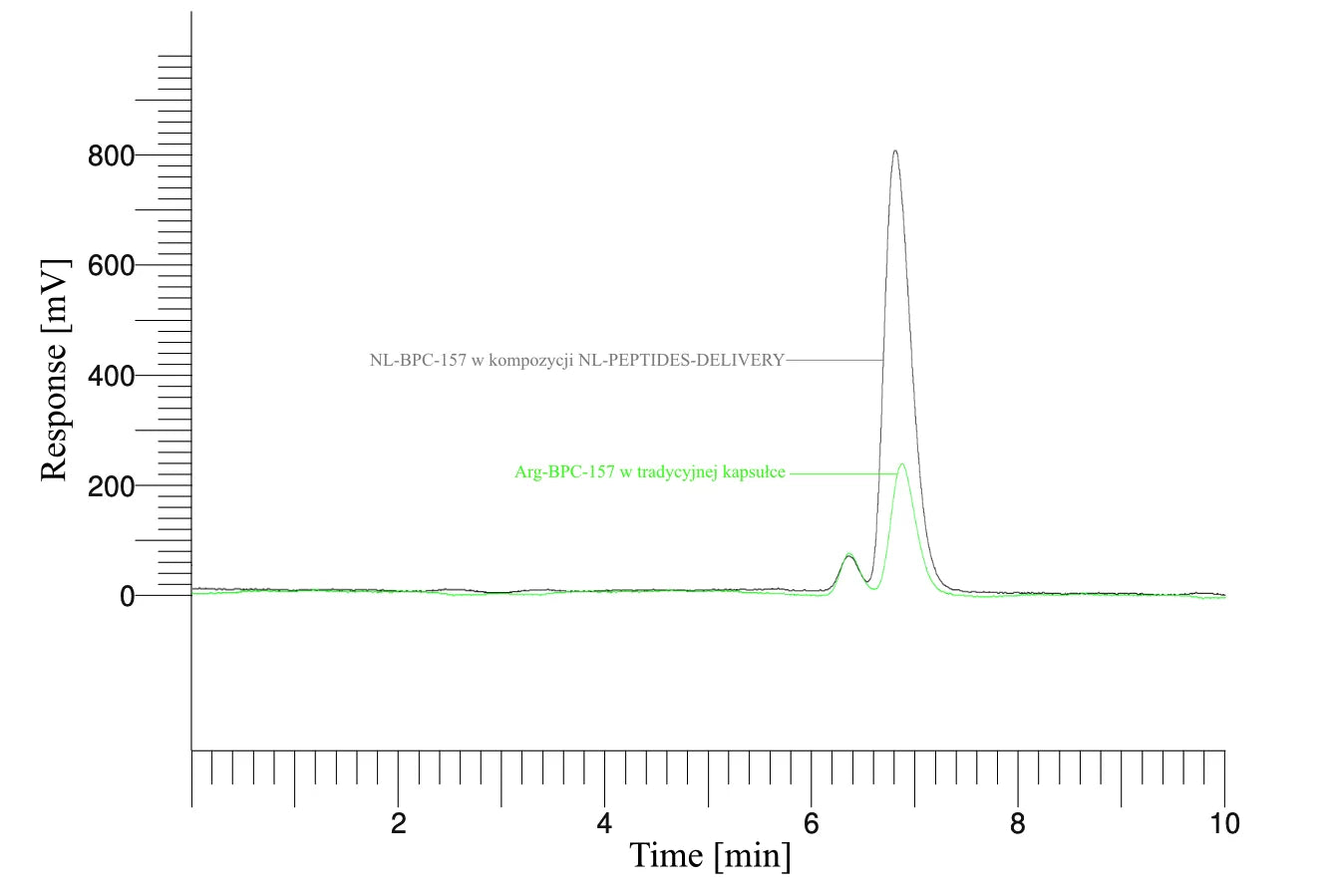
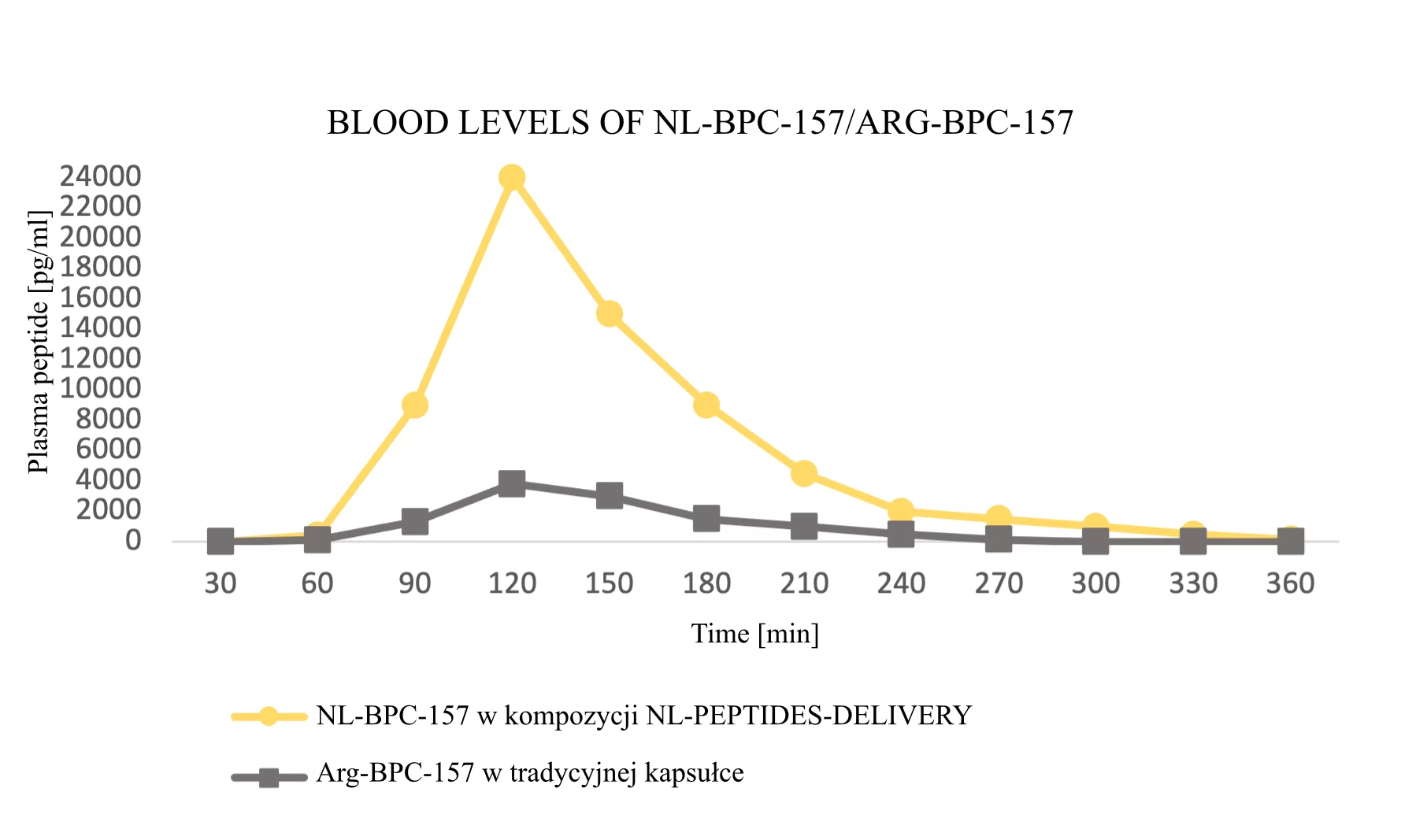
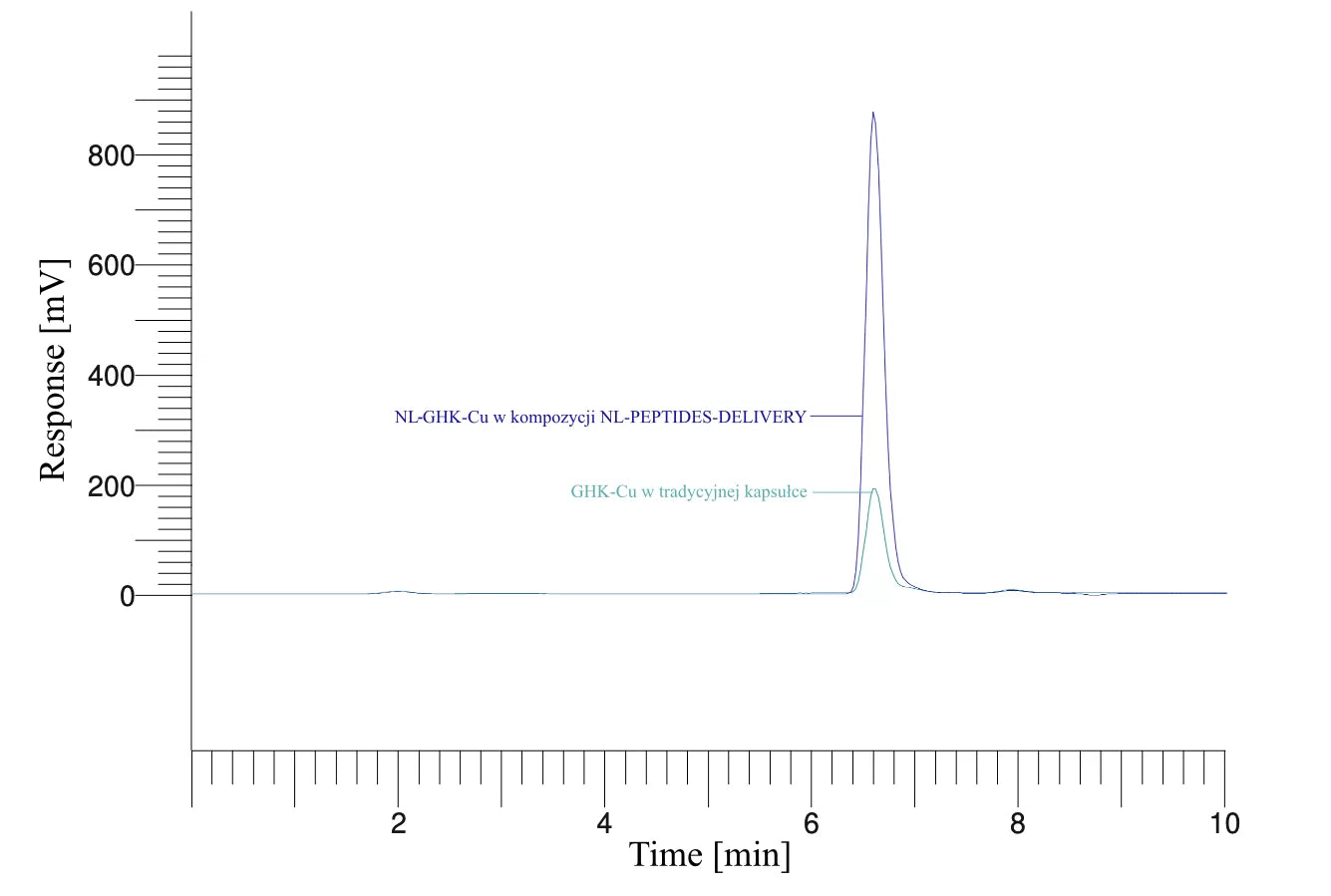
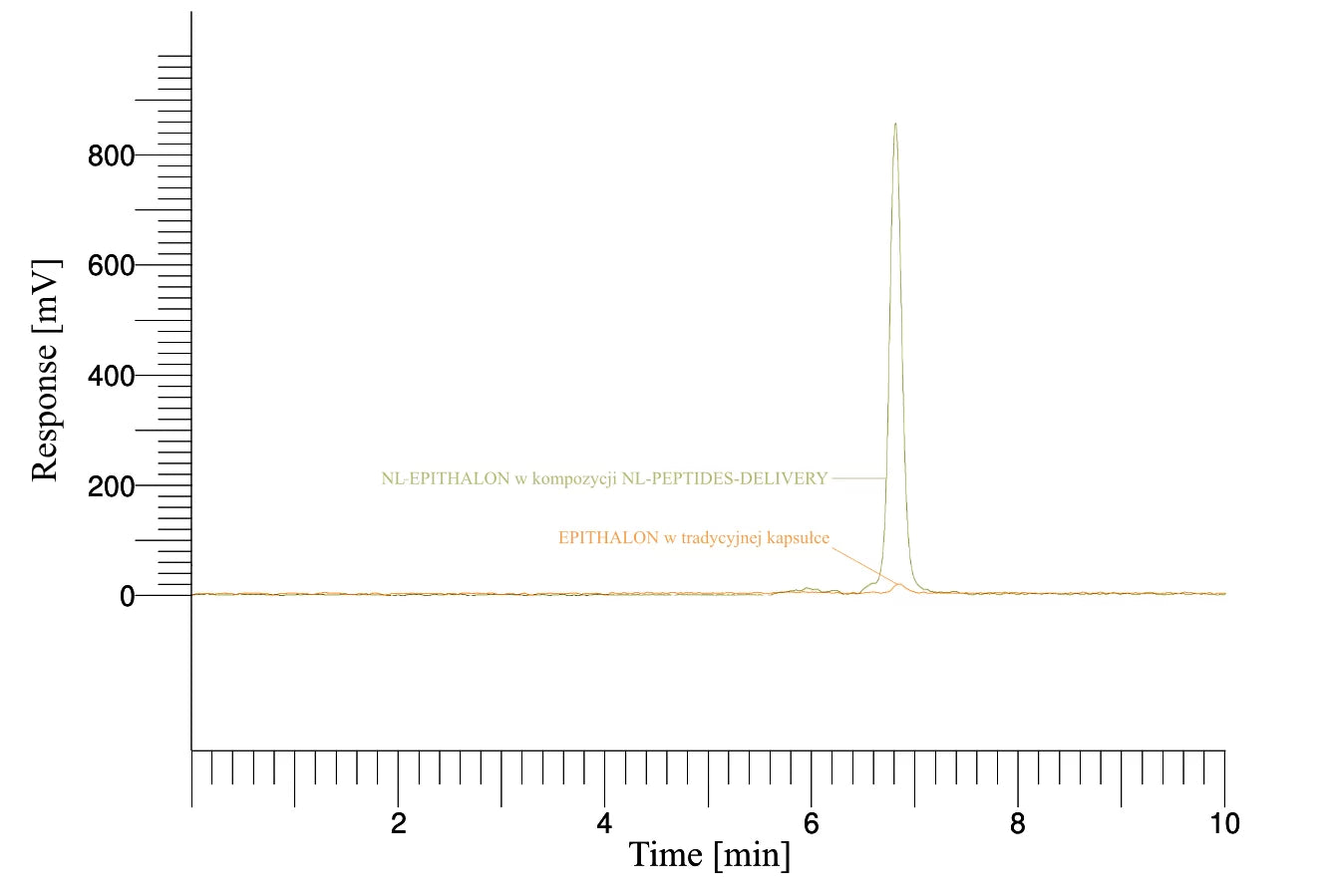
BIOTECHNOLOGICAL RESEARCH – How do our products work?
Source: Characterization and functional assessment of the products NL-GHK-CU, NL-EPITHALON, NL-BPC-157, NL-CORTAGEN, NL-SEMAX, and NL-SELANK.
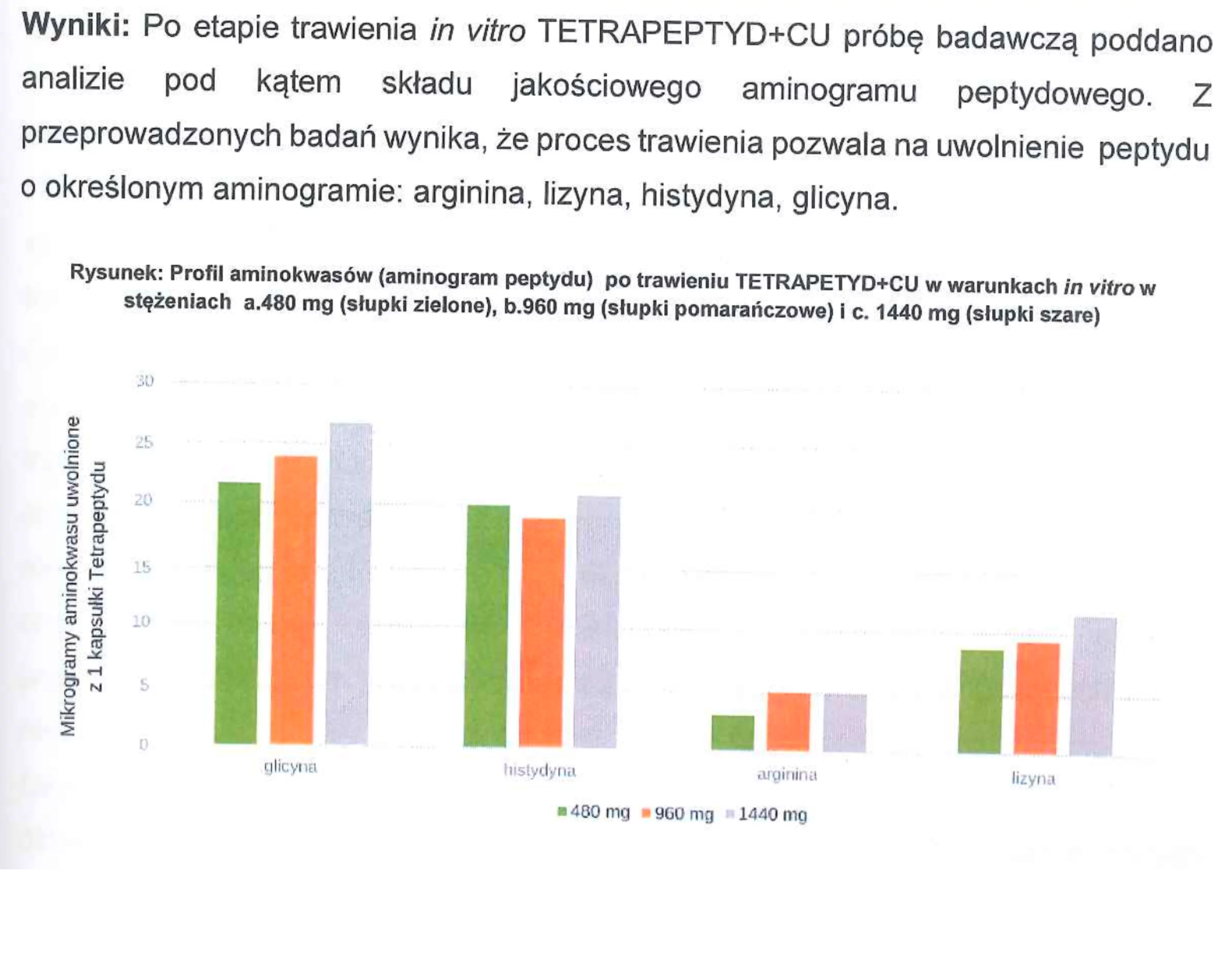
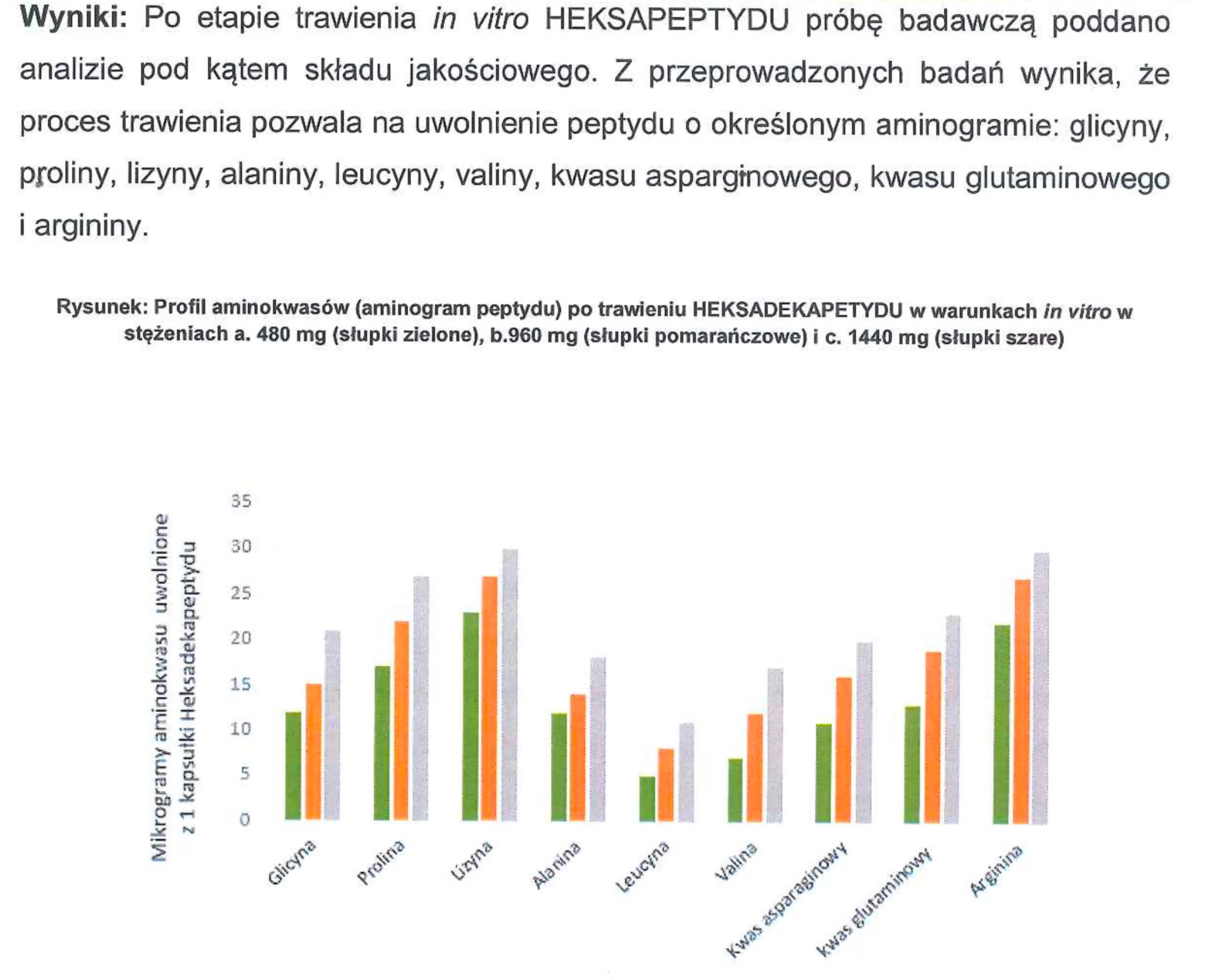
For example: We conducted studies such as the evaluation of the effects of the in vitro digestion process on the active substances contained in the products, along with the assessment of the bioavailability of active ingredients across the intestinal-blood barrier. Additionally, we analyzed the release rate of active substances (peptides) from the products within the gastrointestinal tract, as well as dosage determination and many other parameters.
Conclusion: Our NL-peptides pass completely into the intestine and easily cross the intestinal-blood barrier. Simply put — they work, and their amino acid profile remains unchanged.
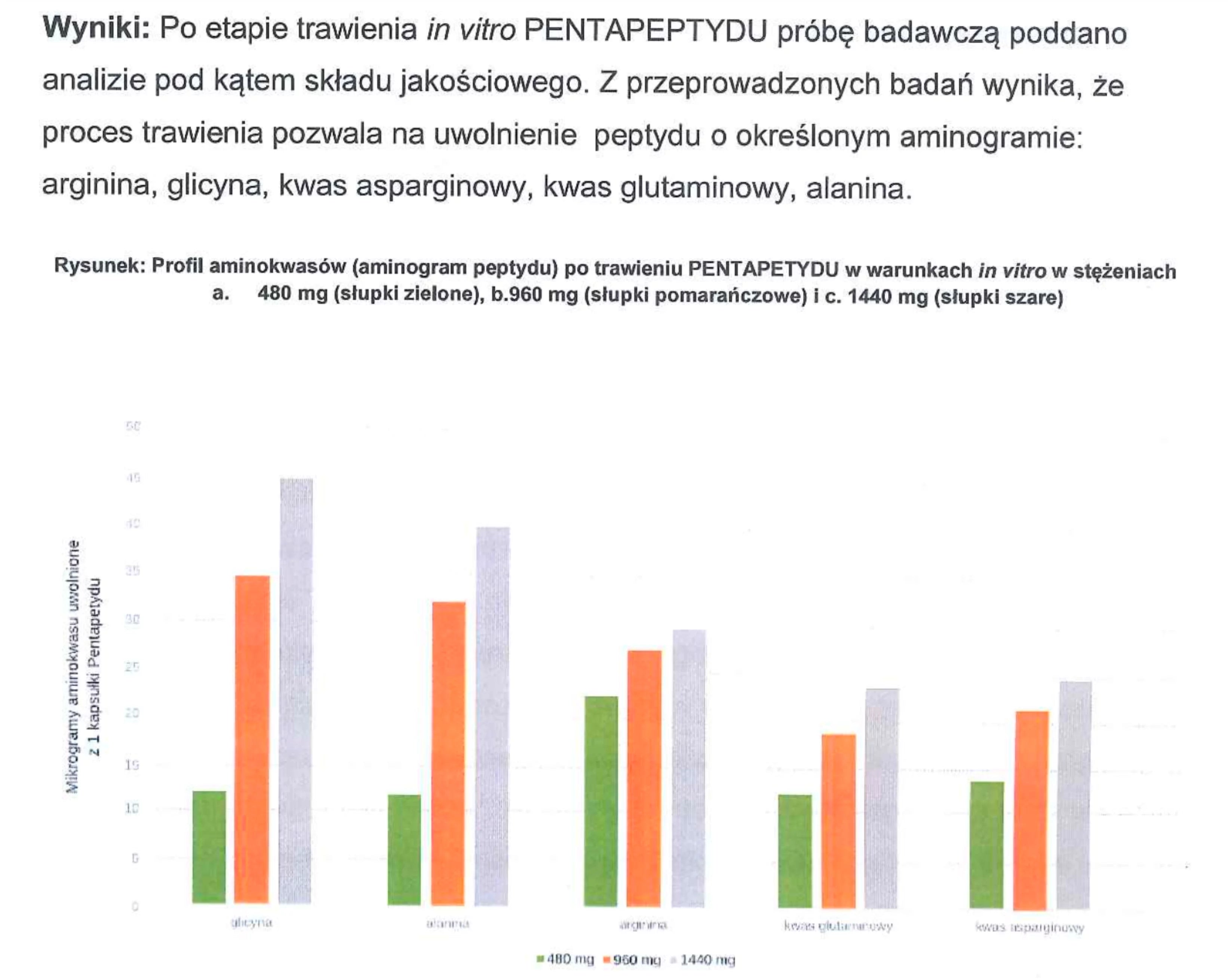
PRODUCT COMPOSITION ANALYSIS – What am I buying?
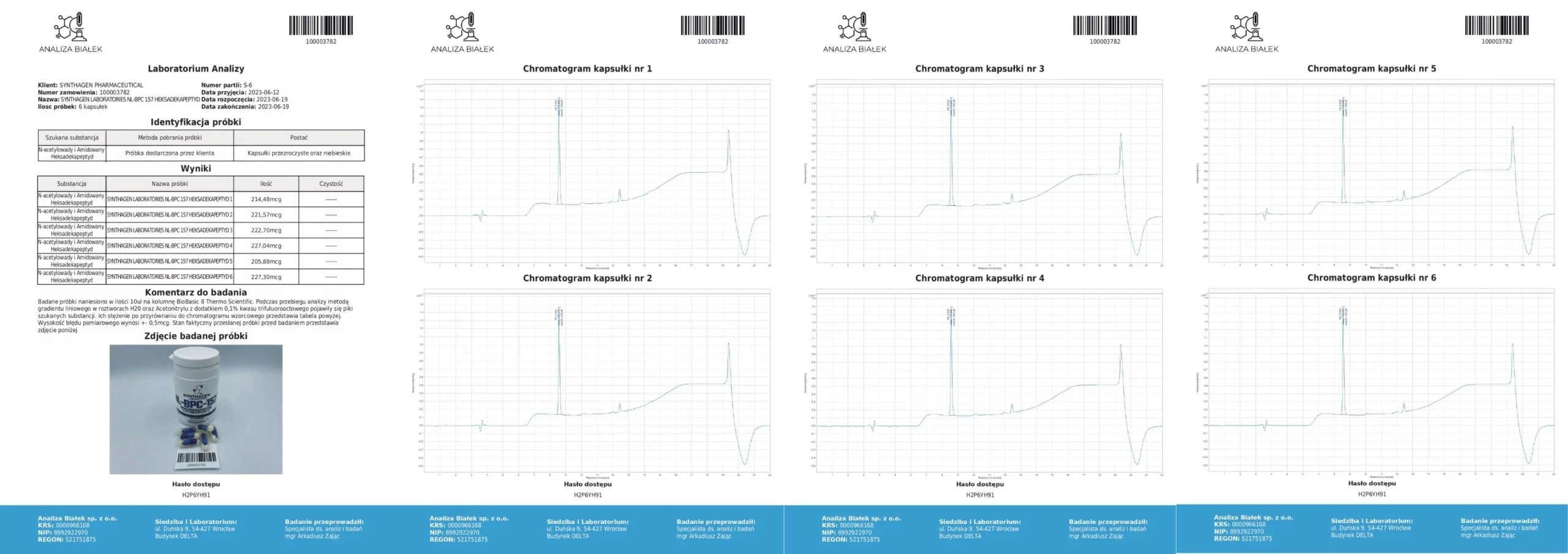
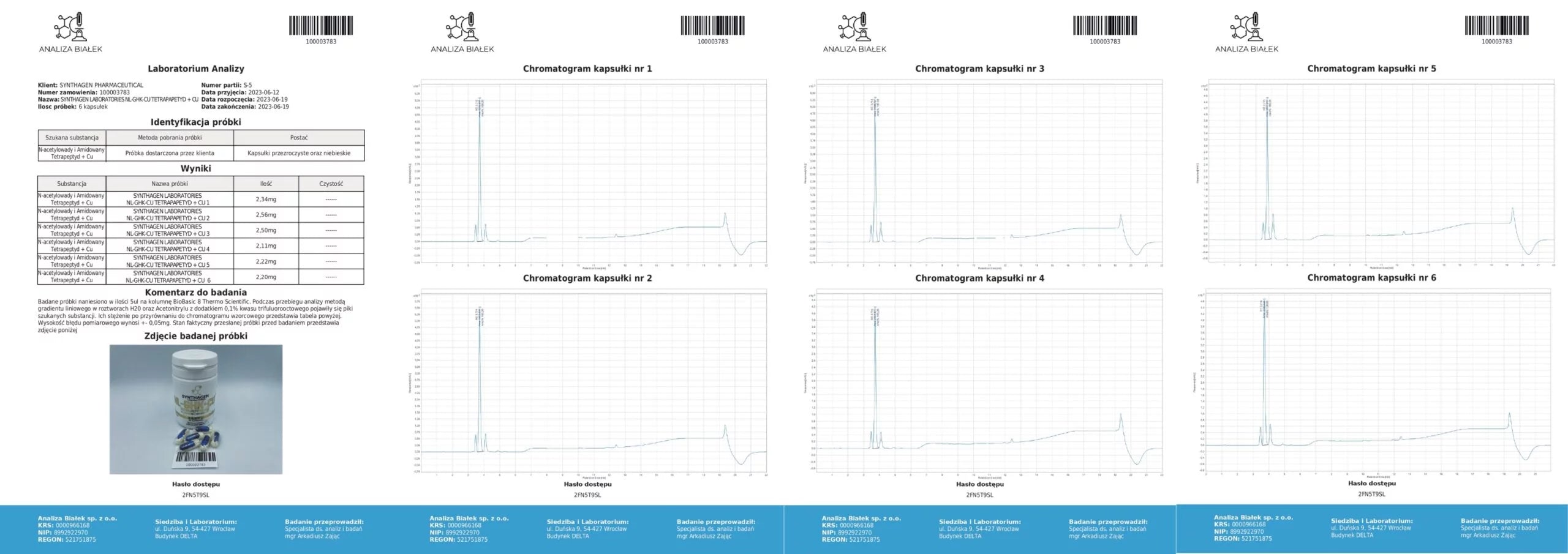
Source: Product composition studies.
Tested samples (15 µl each) were applied onto a BioBasic 8 Thermo Scientific column. During the analysis, using a linear gradient method in H₂O and acetonitrile solutions with 0.1% trifluoroacetic acid, peaks corresponding to the target substances appeared. Their concentrations, compared against the reference chromatogram, are presented in the tables. The measurement error margin is ±0.5 µg.
Conclusion: Our products contain compositions consistent with the declared content.
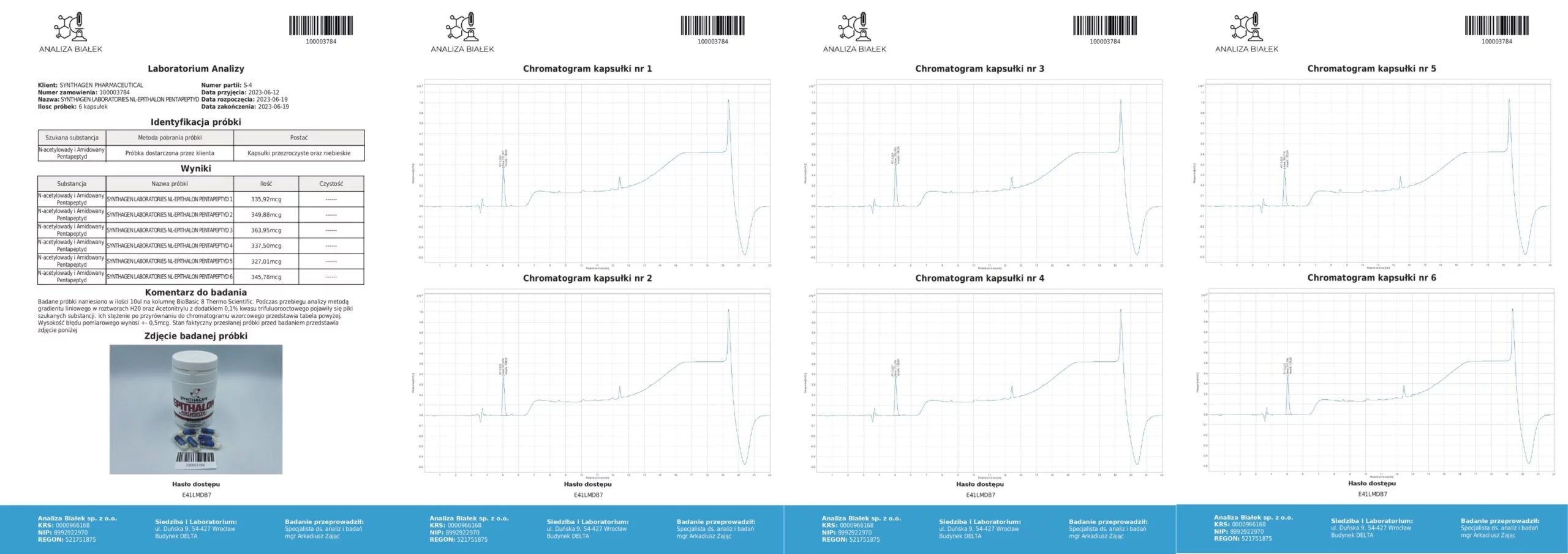
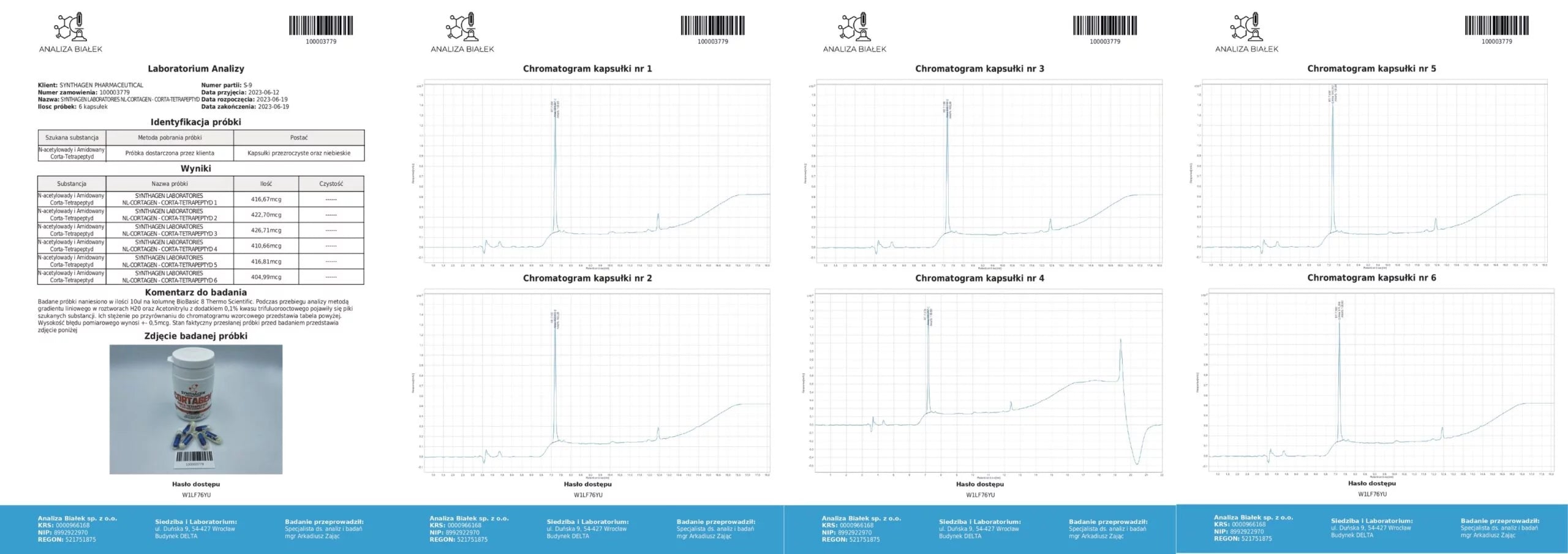
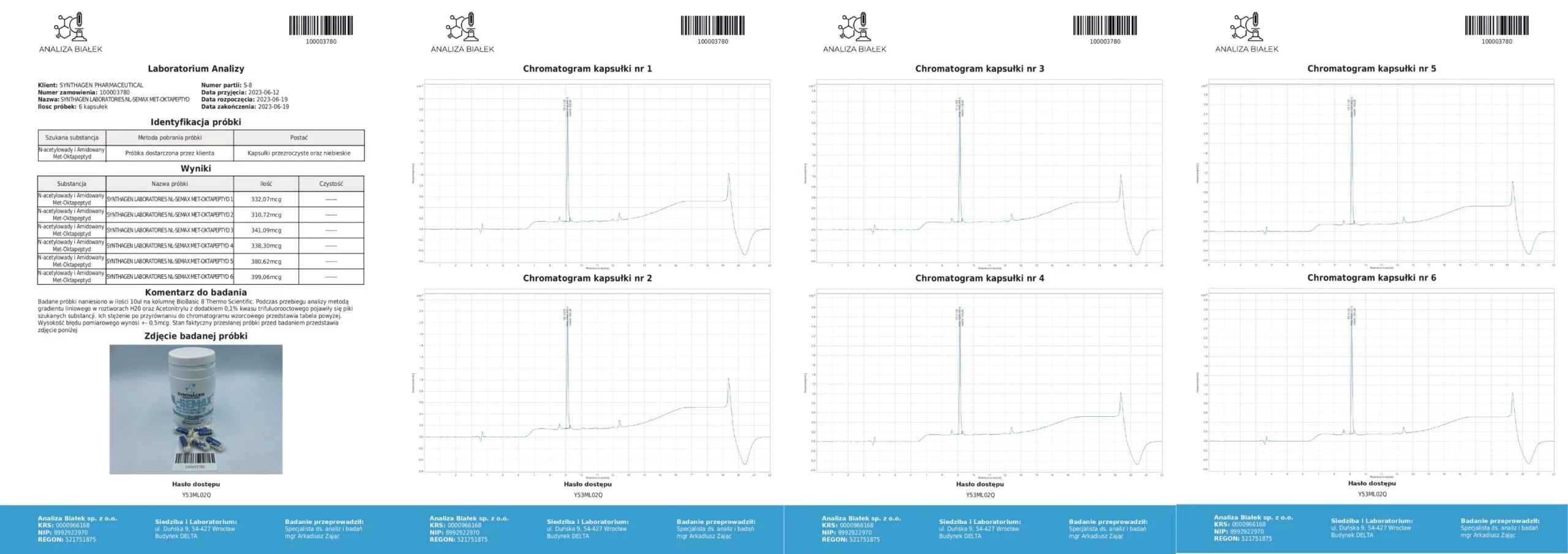
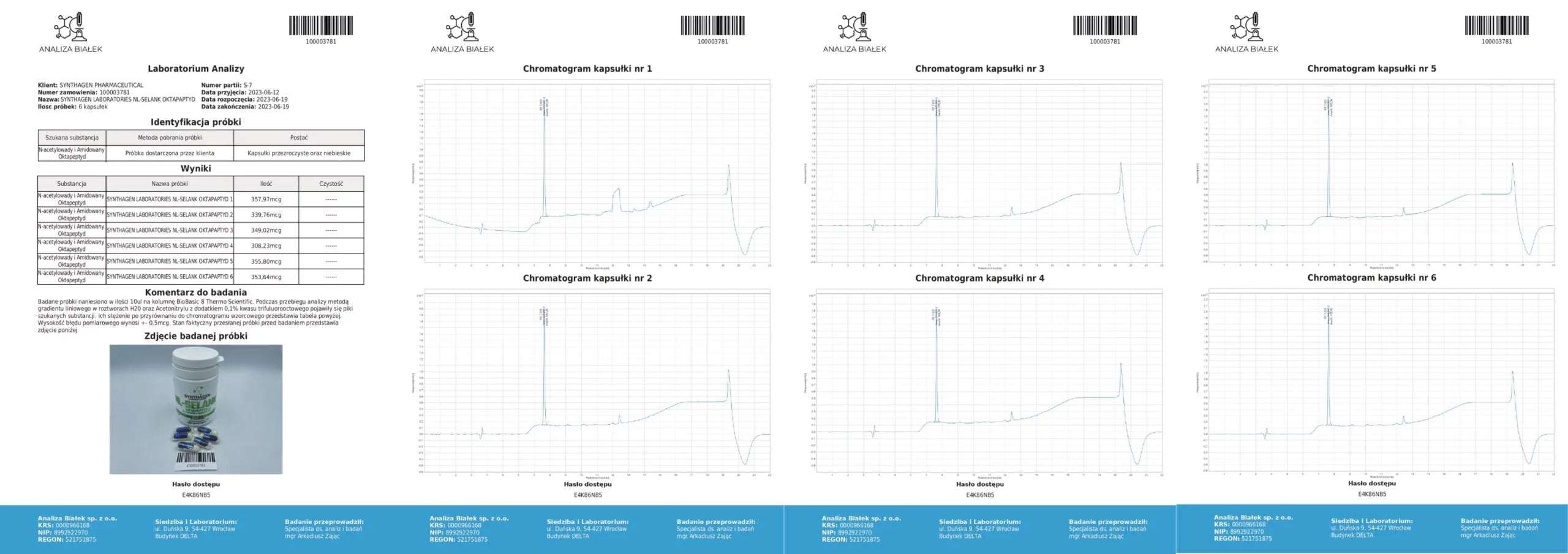
Source: Analytical testing
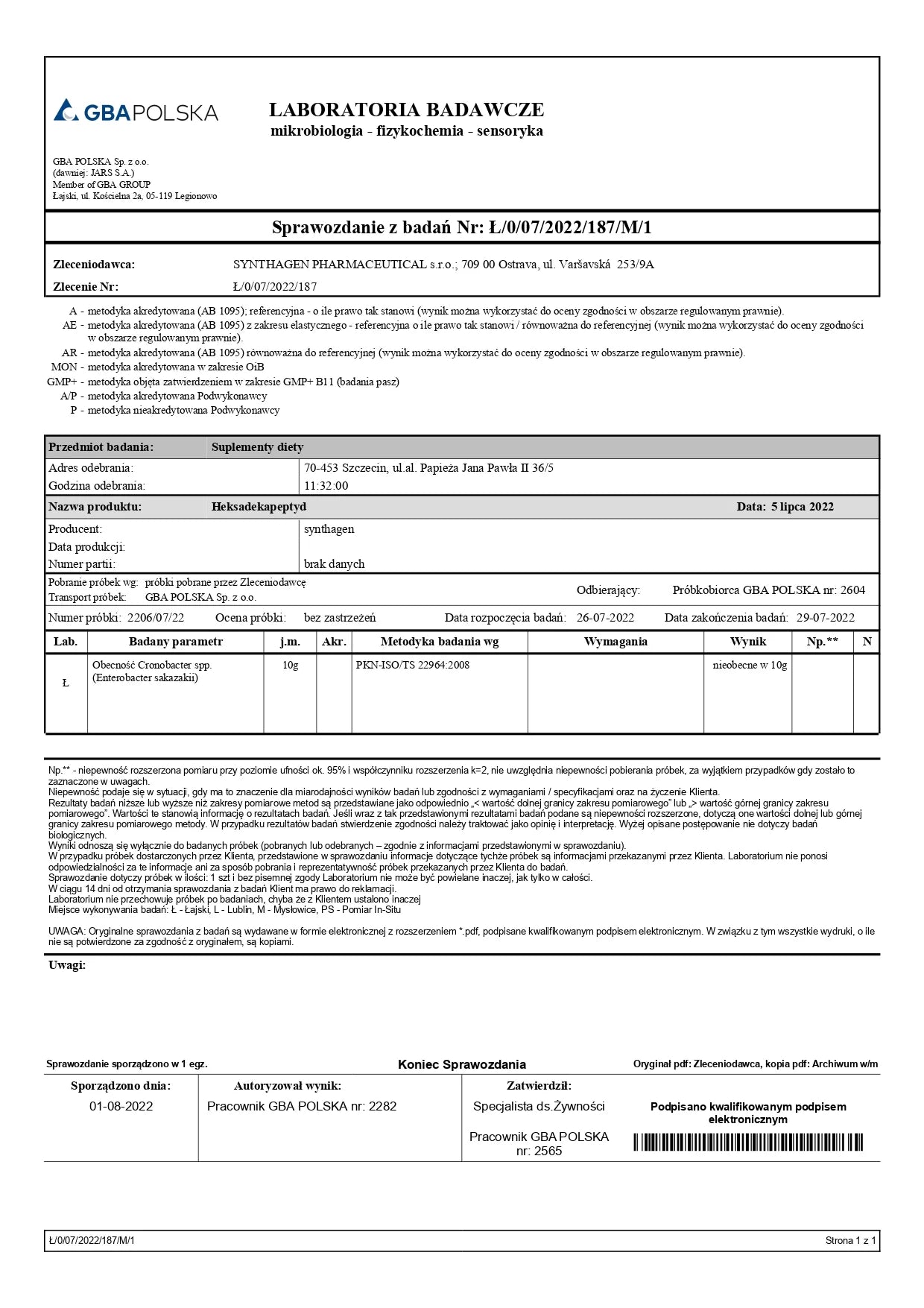
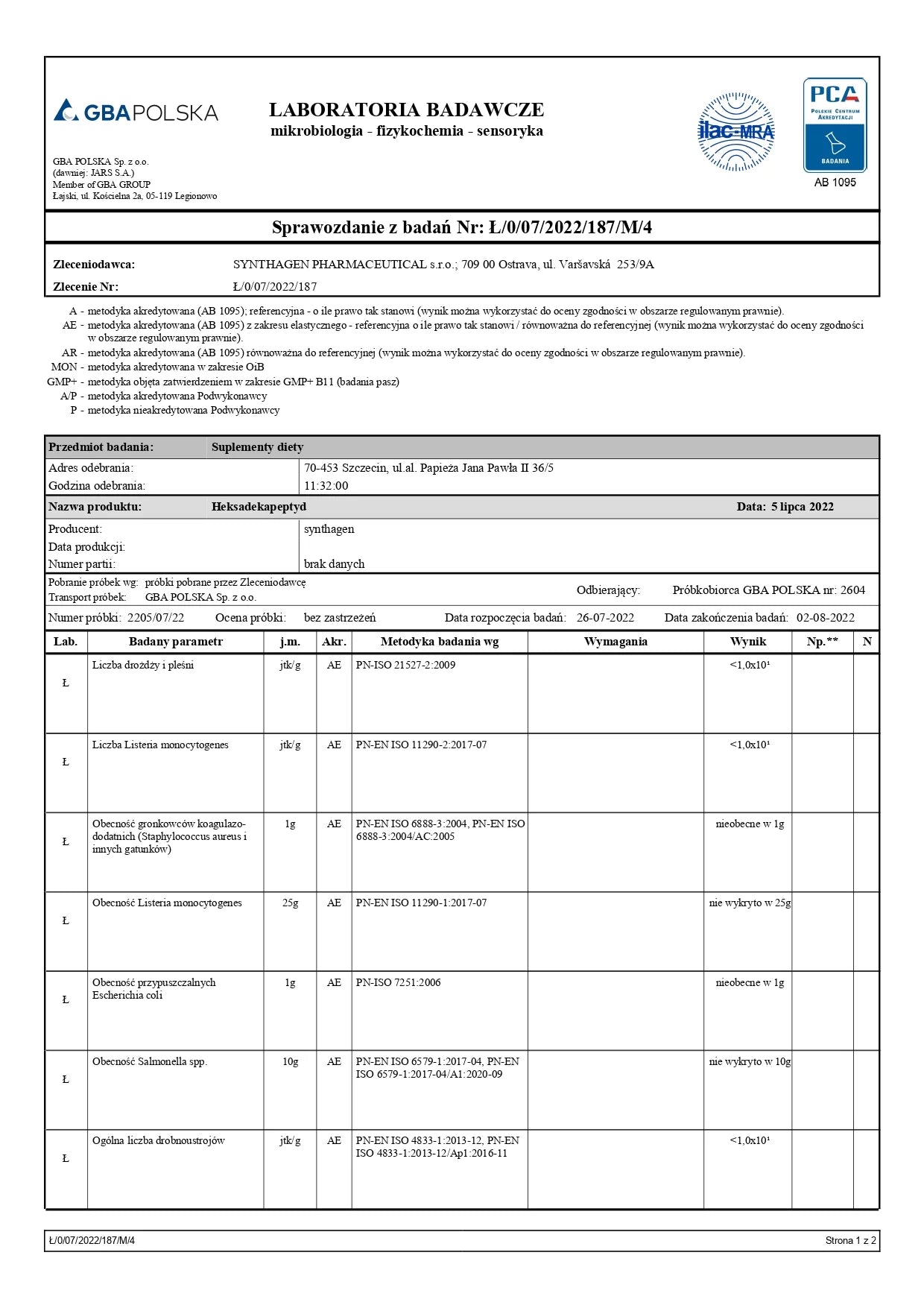
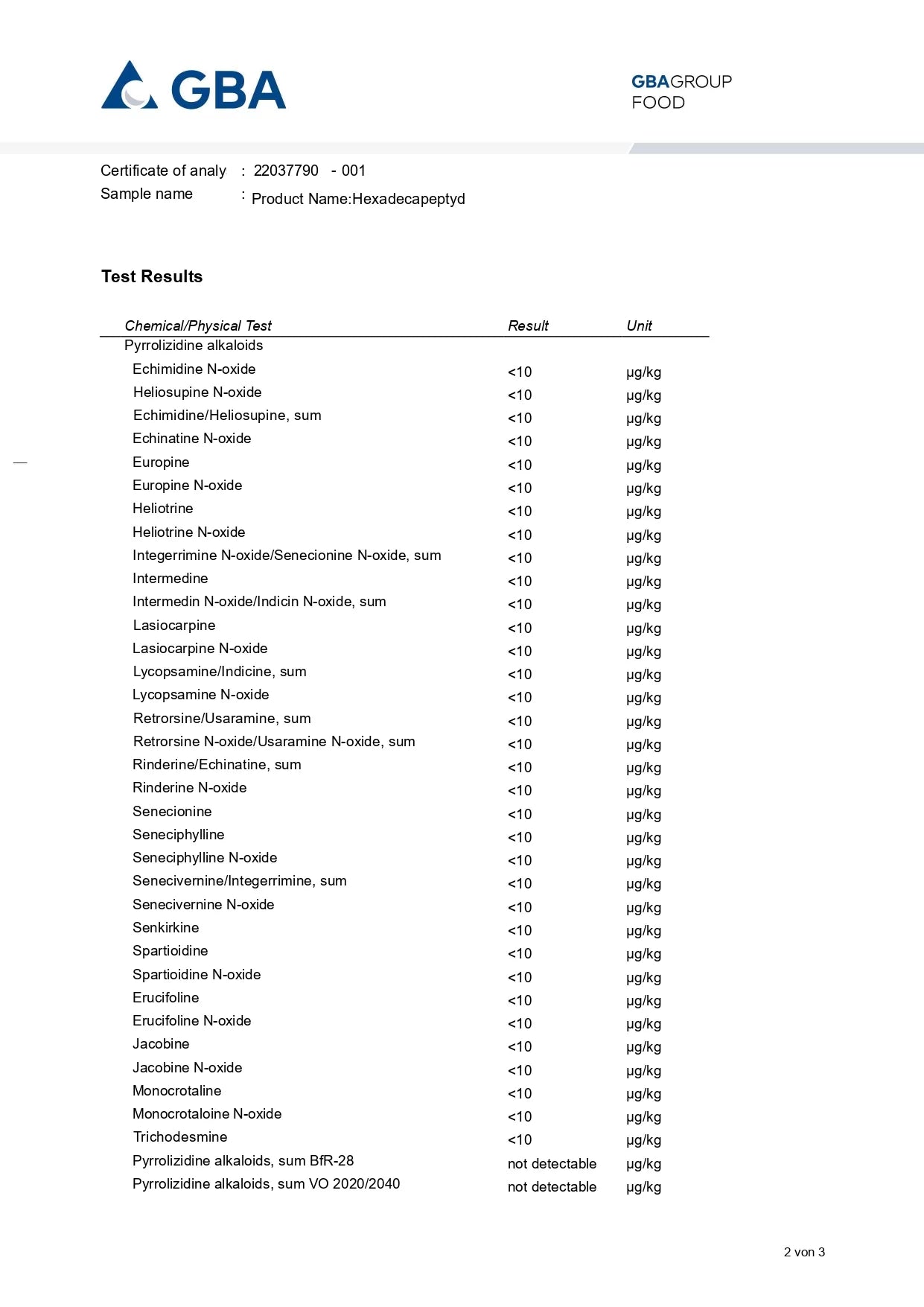
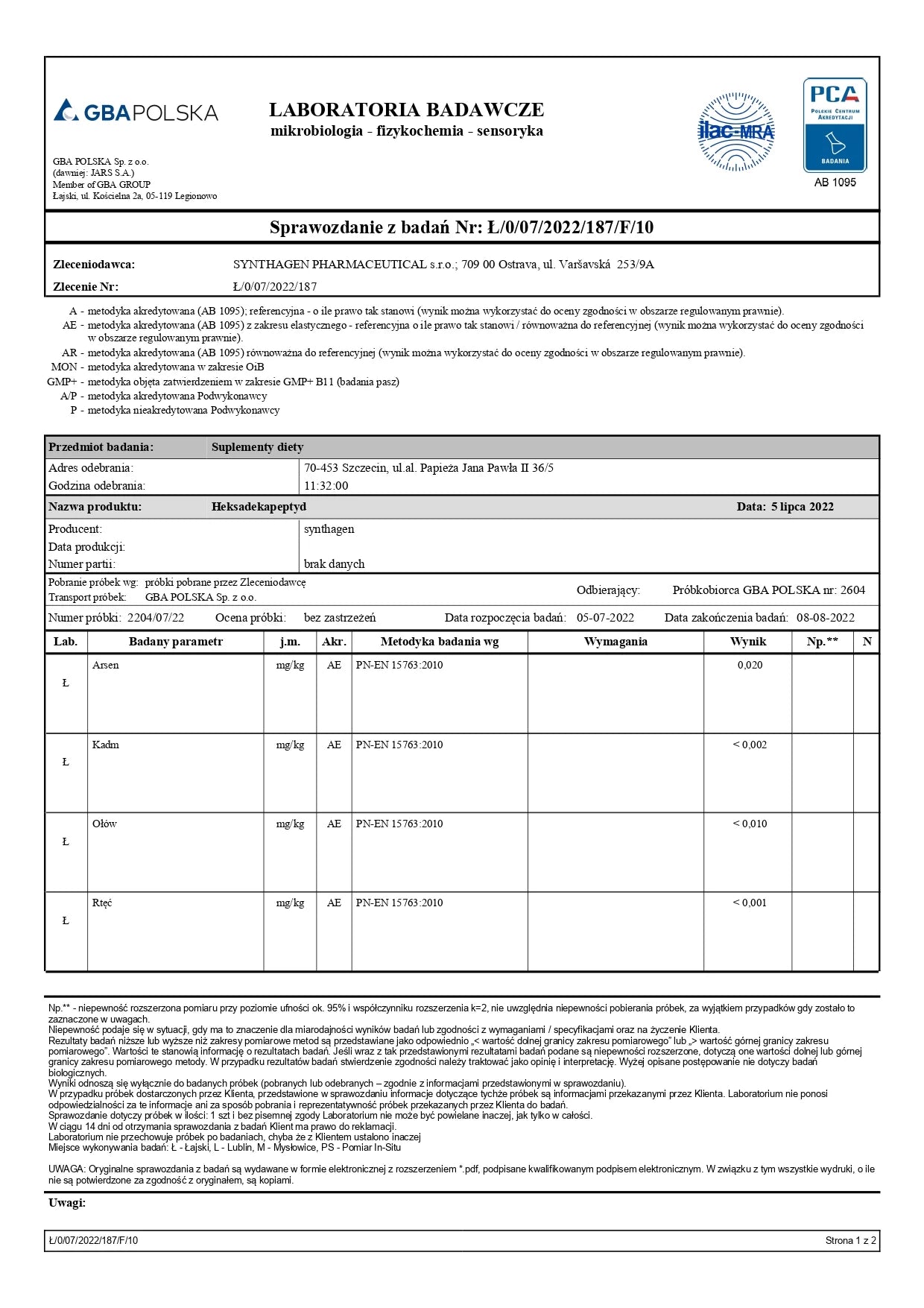
ANALYTICAL TESTING – AM I BUYING SAFE PRODUCTS?
Studies conducted included: presence of Cronobacter, microbiological analysis, alkaloids, heavy metals, and ethylene oxide.
Conclusion: Our products are safe.