Synthagen Laboratories
NL-PEPTIDES DELIVERY™ technology as an innovative method for creating new-generation peptides. Comparison of the action and durability of traditional peptides with the innovative method of peptide synthesis and oral administration. Abstract: In practice, only a few traditional peptides are used due to their biological instability and rapid breakdown. The solution to this problem is peptide modification, which allows for the creation of stable and effective forms of peptides. New-generation peptides, created by synthesis using NL-PEPTIDES technology, are enclosed in a double capsule developed in the NL-PEPTIDES DELIVERY™ system, which maximises the action and stability of the peptide. List of abbreviations: AFP – Actively Pharmaceutical Peptides, NL-PEPTIDES - New generation peptides, NL-PEPTIDES DELIVERY - Oral delivery technology Keywords: peptide, peptide bond, analogue, modification, desalting, amidation, acetylation, synthesis, technology, desalting, arginine
Introduction
NL-PEPTIDES DELIVERY™ technology, as an innovative method, allows for therapeutic effects that exceed those of traditional peptides. The effectiveness of NL-PEPTIDES-DELIVERY™ has been confirmed based on responses to issues related to the production of peptides that reach the intestine intact, where they are then completely absorbed. The innovative NL-PEPTIDES DELIVERY™ technology is protected by patent law as a new form of peptide analogues for the development and commercialisation of oral peptides with a simultaneous, special delivery system.
WHAT ARE TRADITIONAL PEPTIDES?
In purely chemical terms, peptides occur in an unbranched form and have only two specific ends. One of them is called the amino end, where there is an amino acid with a free α-amino group. The other is called the carboxyl end or C end, where there is an amino acid with a free α-carboxyl group. Peptides are chemical compounds similar to proteins, composed of amino acids. They are the subject of widespread interest due to their important biological functions. Many hormones and neurotransmitters are peptides. Endogenous peptides have antimicrobial properties, acting as the body's defence system. Naturally occurring peptides are considered attractive compounds with therapeutic significance due to their high degree of activity, low toxicity and lack of interaction with drugs. In medical practice, only a few peptides are used due to their biological instability and rapid breakdown. The solution to the aforementioned problem of peptide stability is their synthesis, which allows stable peptide forms to be obtained. The same applies to the synthesis of peptides from natural sources, which are used, among other things, in the production of vaccines.
PEPTIDE BINDING
Depending on the peptide we want to obtain, we need an appropriate method for its synthesis. In this brief explanation, we will try to present peptide synthesis in relation to its size. To obtain a dipeptide, use a reagent that activates the carboxyl group of the arylating amino acid or convert the acylating amino acid into an anhydride. A more labour-intensive and difficult process is the synthesis of larger peptides, which we obtain from a dipeptide, where the amino group of the N-terminal amino acid is removed and acylated with another N-protected amino acid. This process is particularly time-consuming because the above steps are repeated until a peptide with the planned sequence is obtained. In the case of obtaining large peptides, the Merifield method is the most effective and easiest method. This method is carried out in the solid phase. The C-terminal amino acid is attached to the polymer, where another amino acid is then attached until the desired chain length is achieved.
PEPTIDE ANALOGUES
In response to the unstable nature of traditional peptides, analogues are being designed and produced. Peptide analogues are chemical compounds in which one atom is replaced by another in relation to the original compound. The overall structure of the peptide remains unchanged. Peptide analogues include helical analogues and β-turn and β-sheet analogues. In the former, helices are one of the key structural elements of bioactive peptides. Stabilising short oligomer fragments in a helical conformation increases activity. In β-bend and β-sheet analogues, D-amino acid or β,γ,δ-amino acid residues are inserted. Peptide analogues allow us to obtain new peptide compounds that are more stable, while finding application in a wider range of symptoms and allowing for innovative solutions to problems associated with the operation of existing pre-analogue forms.
PEPTIDE ANALOGUES THROUGH MODIFICATION
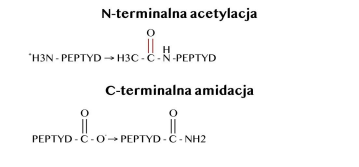
Traditional peptides, despite their undoubted and numerous advantages, also have many limitations related to their use. The search for new, maximally stable peptide analogues with a broad spectrum of activity is a result of the instability of traditional peptides. Analogues of traditional peptides containing a key peptide sequence are enriched with modifications carried out within the peptide chain or the side chain of amino acid residues present in the sequence. The introduction of amidation and acetylation contributes to improved metabolic stability and selectivity. Known examples of modifications, depending on the desired activity profile of the peptide, include N-terminal acetylation, cyclisation, fluorophore labelling, C-terminal amidation, and D-amino acids.
PEPTIDE SALTING OUT PROCESS
The desalting process involves changing protein charges. Protein charges are neutralised by salt anions and cations. Protein molecules do not attract each other and do not form aggregates, and the protein itself is precipitated as a result of the loss of its water coat. The desalting process is reversible. In the reversal process, the salt is removed by dialysis or its concentration is reduced by adding water. Salting out, through the addition of arginine molecules, leads to the formation of a stable form of the peptide and is an innovative method of ensuring peptide stability and, consequently, extending the biological activity of peptides.
ACETYLATION AND AMIDATION OF PEPTIDES
N-terminal acetylation of peptides involves the attachment of acetyl radicals to substrates, which are compounds with NH₂, OH or SH groups, with the participation of the enzyme N-acetyltransferase. The source of the acetyl radical is acetyl-CoA. The main function of N-acetyltransferases is to facilitate the connection of the acetyl group with the amino group of aromatic amines and hydrazines (N-acetylation reaction), i.e. the detoxification of potentially toxic exogenous compounds. When peptide bonds are broken and the polypeptide chain is fragmented, carbonyl groups are formed. The oxidation of a protein molecule by a hydroxyl radical begins with the detachment of a hydrogen atom from the α carbon of an amino acid. The resulting alkyl radical reacts with oxygen to form an alkyl peroxide radical, which is converted into an alkyl hydroperoxide. The alkoxyl radical formed from it can be converted into a hydroxylated amino acid residue at the α carbon or can lead to fragmentation of the polypeptide chain. The presence of an alkoxyl radical promotes fragmentation of the polypeptide chain. Peptide bond cleavage can occur via α-amidation or diamidation. The N-terminal peptide formed during α-amidation fragmentation has an amide group at the C-terminus, while the other peptide contains an N-α-ketoacyl derivative at the N-terminus. Fragmentation via the diamide pathway is characterised by the formation of an N-terminal peptide containing a diamide structure and a peptide derived from the C-terminal end of a protein molecule containing an isocyanate structure at the N-terminal end.

WHAT ARE NL-PEPTIDES™?
In response to issues concerning the production of peptides that reach the intestine intact, we have created a group of new peptides called NL peptides, also referred to as new-generation peptides, marked with the abbreviation AFP. The characteristics of pharmaceutically active peptides reflect their advantage over traditional peptides. They are primarily characterised by extended shelf life and resistance to changes in pH and temperature, both during product storage and in the digestive system, where the peptide will not degrade when the capsule is ingested and transported to the small intestine. The NL-peptide group is characterised by its ability to mimic naturally occurring proteins and high metabolic stability, thanks to which NL-peptides, acting like natural proteins, are absorbed intact from the intestine and do not degrade during absorption.
THE FORMATION OF NL-PEPTIDES™ THROUGH SYNTHESIS
The chemical representation of NL-peptides, created through modifications by synthesis, will be discussed below. The technology, which works on the principle of salting out the peptide with arginine, has been enhanced by synthesis through N-acetylation of the amino end of the peptide with simultaneous amidation of the carboxyl end of the bond. All of the modification processes carried out result in a 10-fold increase in the stability of NL-PEPTIDES and the aforementioned ability to mimic natural proteins.
NL-PEPTIDES DELIVERY™ TECHNOLOGY
When undertaking the task of creating a modern technology for oral peptide delivery, it was important to solve problems related, first of all, to the creation of a simple technology based on versatility in relation to a wide range of products, as well as the simultaneous production of several effective products at the same time, thanks to a method that enables the development of a system allowing for such a scheme of operation. Previous problems related to absorption were solved by using the aforementioned peptide modifications alone. In our case, in addition to peptide modification, we focused on the undoubtedly important solution to the problem of peptide transport from the moment of oral administration, through the small intestine, until the moment of absorption of the compound. The solution to these technological problems is the creation of a new, simple and effective peptide delivery technology, which we refer to as NL-PEPTIDES-DELIVERY™.
PROFILE OF THE NL-PEPTIDES DELIVERY™ TECHNOLOGY
The action profile, based on NL-PEPTIDES DELIVERY technology, primarily allows the peptide to reach the small intestine through a specially designed coating surrounding the NL-peptide. In addition to the peptide reaching the small intestine, it is important that the entire local environment in the intestines is conducive to the absorption of the peptide delivered to this location. This was a significant problem due to the presence of peptidase enzymes in the intestine, which degrade peptides. The main enzymes that degrade peptides are proteolytic enzymes, called proteases, which prevent the peptide from continuing its journey by degrading it in the small intestine. Another problem we solved was the poor absorption of the peptide itself by the wall of the small intestine.
ADVANTAGES OF NL-PEPTIDES DELIVERY™ TECHNOLOGY
Focusing on the specific profile of NL-PEPTIDES DELIVERY™ technology, it involves the development of a new double capsule with protective coatings, which solves the problem of both the peptide reaching the small intestine and passing through the intestinal wall, as well as the degradation of the peptide at this point. The protective coating, which forms the outermost surface of the capsule, shields the compound from pH changes and stomach acid, allowing the peptide to reach the intestine. Thanks to the use of a protease inhibitor in the capsule, the peptide, which is susceptible to degradation, does not break down, while lowering the local pH of the intestine and preparing its environment for peptide absorption. An absorption enhancer has been used to improve the absorption of the peptide by the small intestine. The NL peptide is surrounded by a coating that separates it from the protease inhibitor. The inhibitor itself is an acid which, when combined with the peptide, could degrade it. By separating it from the acid using a capsule within a capsule, we have obtained a stable pharmaceutical composition that does not degrade during storage.
HOW NL-PEPTIDES DELIVERY™ TECHNOLOGY WORKS IN THE DIGESTIVE SYSTEM?
New-generation peptides, created through synthesis using NL-PEPTIDES technology. Encapsulated in a double capsule created in the NL-PEPTIDES DELIVERY™ system, which, when administered orally, follows a specific path in the digestive system. Once swallowed, the capsule reaches the small intestine intact, where the protease inhibitor is released, creating the right environment for absorption by lowering the pH of the intestine to 5.5. In the next stage, the active ingredient and absorption enhancer are released, allowing the peptide to be effectively absorbed by the body.
RESULTS OF USING NL-PEPTIDES DELIVERY™ TECHNOLOGY
The concentration of the peptide in the blood after ingestion of the double capsule we have created is presented in the form of the graph below, where the result obtained clearly confirms the presence of NL-peptide in the blood. This means that the peptide has reached and been absorbed, and consequently is acting in the body. The first three sample graphs show the peaks of peptides from appropriately prepared blood samples tested at specific times. Sequentially for: NL-GHK-CU, NL-EPITHALON and NL-BPC-157 administered using our patented NL-PEPTIDES-DELIVERY TM technology, and for regular GHK-CU, EPITHALON and ARG-BPC peptides administered in regular capsules. The next three graphs show the distribution of peptide concentrations in the tested blood over a time range of 30-360 minutes, converted to pg/ml units. A comparison of the results of oral administration in the form of a double capsule with the administration of the peptide in the form of nasal administration allows us to determine the superiority and effectiveness of one of the administration methods. As shown in the graph, the concentration of the peptide at the time of oral administration of our capsule is much higher than the concentration of the peptide administered intranasally. The same doses of the peptide were used in the capsule and in the nasal spray during the study. The results of these studies demonstrate the superiority of our technology over intranasal administration.
THE EFFECT OF SYNTHAGEN LABORATORIES' WORK
The task we set ourselves was to create a new technology that would increase the effectiveness of peptides. The NL-PEPTIDES-DELIVERY oral peptide delivery technology is based on innovative peptide therapy combined with a novel oral delivery method developed by us. A multi-channel presentation of our technology allows for a thorough understanding of each of its stages. The effects of our work can be seen in the presentation.





Peptides with antimicrobial properties and their analogues created through modification.
The role of the NL GHK-Cu peptide in skin and hair restoration and regeneration processes